A 0.0240 mol sample of N 2 O 4 (g) is allowed to come to equilibrium with
Question:
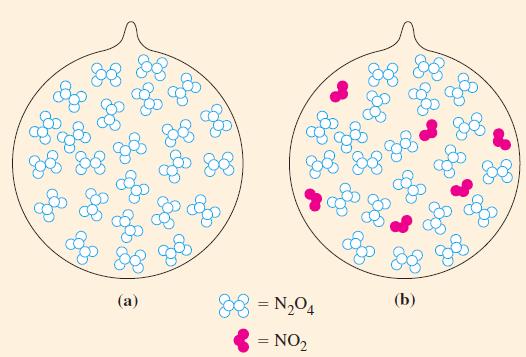
A 0.0240 mol sample of N2O4(g) is allowed to come to equilibrium with NO2(g) in a 0.372 L flask at 25 °C. Calculate the amount of N2O4 present at equilibrium (Fig. 15-9).
![]()
Figure 15-9
Transcribed Image Text:
N₂O4(g) 2 NO₂(g) Kc = 4.61 × 103 at 25 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Analyze We need to determine the amount of N 2 O 4 that dissociates to establish equilibrium For the ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) If 0.150 mol H 2 (g) and 0.200 mol I 2 (g) are introduced into a 15.0 L flask at 445 C and allowed to come to equilibrium, how many moles of HI(g) will be present? (B) Suppose the equilibrium...
-
A 1.100 L flask at 25 C and 1.00 atm pressure contains CO 2 (g) in contact with 100.0 mL of a saturated aqueous solution in which [CO 2 (aq)] = 3.29 x 10 -2 M. (a) What is the value of K c at 25 C...
-
A.0.50 mole sample of N2O4(g) is allowed to come to equilibrium with NO2(g) in a 0.750L flask. At equilibrium , 0.42mol N2O4 remains. Calculate the equilibrium concentrations of N2O4 and NO2 and...
-
The following trial balance is taken from the General Fund of the City of Jennings for the year ending December 31, 2017. Prepare a condensed statement of revenues, expenditures, and other changes in...
-
Valley Corporation is attempting to select the best of a group of independent projects competing for the firms fixed capital budget of $4.5 million. The firm recognizes that any unused portion of...
-
Charles and L. W. Clement were brothers who had formed a partnership that lasted forty years until Charles discovered that his brother, who kept the partnerships books, had made several substantial...
-
Choose a country from three of the regions presented in Table 6.7. Using the Internet, collect as much information as you believe is needed to identify the potential for market segments based on age,...
-
Ling, an audit manager, is planning the audit of Modern Technologies, Inc., (MT, Inc.) a manufacturer of electronic components. This is the first year that Lings audit firm has performed the audit...
-
A single nuclear reactor produces 2.6GW of electrical power, and has a generator voltage of 22kV.What percentage of power would be lost from our nuclear reactor in three-line transmission that goes...
-
Given the equilibrium constant values 1 N2(g) + O2(g) = NO(g) K = 2.7 10-18 2 NO2(g) Kc = 4.6 x 10- Kc = 4.1 10- N2O4(g) N2(g) + O2(g) NO(g) N2(g) + O2(g) 2 Determine a value of K, for the...
-
Determine K c for the reaction 2 N2(g) + O2(g) + Br2(g) NOBr(g) from the following information (at 298 K). 2 NO(g) 1 Br(g) NO(g) + 2 N2(g) + O(g) K = 2.1 10 NOBr(g) K = 1.4
-
1. Electricity is a service that customers rarely think about until there's a power outage. 2. How can BC Hydro utilize digital media to gain support for its proposed rate increase? 3. Discuss the...
-
A box of textbooks of mass 27.7 kg rests on a loading ramp that makes an angle. with the horizontal. The coefficient of kinetic friction is 0.25 and the coefficient of static friction is 0.37. Part A...
-
How did Rome, a small settlement in central Italy, expand to conquer and control the entire Mediterranean world of Europe, western Asia, and North Africa? What allowed Rome to retain such a large...
-
Henrico County (suburban Richmond, Virginia) is in the process of constructing a 17,000-seat arena and convention center that will have accompanying commercial developments, condos, apartments, etc....
-
SQL Server uses what model? Document O Transactional Object-oriented O None of these Normalized
-
How might companies deal with unstable times and retain talent?
-
Aunt Ethel's Fancy Cookie Company manufactures and sells three flavors of cookies: macaroon, sugar, and butter cream. The batch size for the cookies is limited to 1,000 cookies based on the size of...
-
Find the equation of the plane passing through the points P 5,4,3 ,Q 4,3,1 and R 1,5,4
-
The consulting firm of Tilton and Henderson accumulates costs associated with individual cases, using a job order cost system. The following transactions occurred during June: June 4. Charged 600...
-
The Ad Guys Inc. provides advertising services for clients across the nation. The Ad Guys is presently working on four projects, each for a different client. The Ad Guys accumulates costs for each...
-
Keltner Co. uses a job order cost system. The following data summarize the operations related to production for November: a. Materials purchased on account, $350,000. b. Materials requisitioned,...
-
Assume a mortgage loan has an outstanding principal balance of $100,000 and the interest rate is 6%. Calculate what portion of a $599.55 monthly payment will be allocated to interest.?
-
Find a website that you like and one that you do not like, which are respectful and abide by Saint Leo University ethics. For each website, identify who the target audience is, how it is organized...
-
Suppose we want to know the electricity emissions profile for any county? Or suppose we're curious about the per capita generation and emissions in each eGRID subregion, but we need to estimate the...

Study smarter with the SolutionInn App


