A pycnometer (see Exercise 78) weighs 25.60 g empty and 35.55 g when filled with water at
Question:
A pycnometer (see Exercise 78) weighs 25.60 g empty and 35.55 g when filled with water at 20 °C. The density of water at 20 °C is 0.9982 g/mL. When 10.20 g of lead is placed in the pycnometer and the pycnometer is again filled with water at 20 °C, the total mass is 44.83 g. What is the density of the lead in grams per cubic centimeter?
Exercise 78
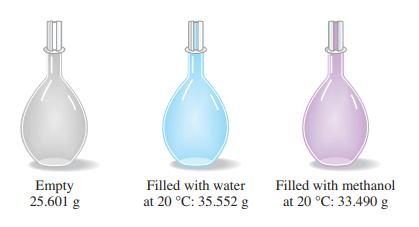
The simple device pictured here, a pycnometer, is used for precise density determinations. From the data presented, together with the fact that the density of water at 20 °C is 0.99821 g/mL, determine the density of methanol, in grams per milliliter.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





