Acetylene (C 2 H 2 ) torches are used in welding. How much heat (in kJ) evolves
Question:
Acetylene (C2H2) torches are used in welding. How much heat (in kJ) evolves when 5.0 L of C2H2 (d = 1.0967 kg/m3) is mixed with a stoichiometric amount of oxygen gas? The combustion reaction is
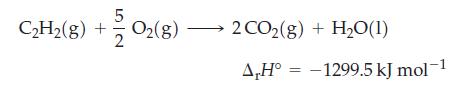
Transcribed Image Text:
5 C₂H₂(g) + +2/202(8) 2 CO2(g) + H₂O(1) A,H° -1299.5 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
First lets balance the chemical equation to make sure we know how many moles of reactants and pr...View the full answer

Answered By

Sandhya Sharma
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
119+ Reviews
214+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Nitric acid is used extensively for the production of inorganic and organic nitrates, for metal treatments of various kinds, and for photoengraving. It is produced by oxidizing ammonia to nitric...
-
A flow of hydrogen gas is mixed with a flow of oxygen in a stoichiometric ratio, both at 298 K and 50 kPa. The mixture burns without any heat transfer in complete combustion. Find the adiabatic flame...
-
A Claus plant converts gaseous sulfur compounds to elemental sulfur, thereby eliminating emission of sulfur into the atmosphere. The process can be especially important in the gasification of coal,...
-
A random sample of 100 students was taken from a large university to study the relationship between GPA and the number of hours of study per week. The following linear regression equation was...
-
Three inventory categories are reported on a manufacturing companys balance sheet: (a) Raw materials, (b) Goods in process, and (c) Finished goods. Identify the usual order in which these inventory...
-
Stadt Corporation of the Netherlands is a 100 percent-owned subsidiary of Port Corporation, a U.S. firm, and its functional currency is the U.S. dollar. Stadts books of record are maintained in euros...
-
Discuss the uses and limitations of the statement of financial position for decision-making purposes.
-
1. Using the rules of thumb for reducing the risks related to introducing an innovative new product, how well are Teal and Pate likely to do with the ElliptiGo? 2. What are the primary benefits and...
-
Prepare a single, continuous multiple-step statement of comprehensive Income for 2021, Including appropriate EPS disclosures. (Round EPS answer to 2 decimal places.) LINDOR CORPORATION Statement of...
-
Substance A in a liquid reacts to produce R and S as follows: A feed (C A0 = 1, C R0 = 0, C S0 = 0) enters two mixed flow reactors in series, ( 1 = 2.5 min, 2 = 5 min). Knowing the composition in...
-
The heat of neutralization of HCl(aq) by NaOH(aq) is -55.84 kJ/mol H 2 O produced. If 50.00 mL of 1.05 M NaOH is added to 25.00 mL of 1.86 M HCl, with both solutions originally at 24.72 C, what will...
-
Refer to Example 7-4. The product of the neutralization is 0.500 M NaCl. For this solution, assume a density of 1.02 g/mL and a specific heat capacity of 4.02 J g -1 C -1 . Also, assume a heat...
-
Astronomers call a shift in the spectrum of galaxies a "redshift." A correlation between redshift level and apparent magnitude (i.e., brightness on a logarithmic scale) of a quasi-stellar object was...
-
Identify from Cresseys research the six situational categories that cause nonshareable problems.
-
What was Cynthia Coopers position at WorldCom?
-
This exercise was also contributed by Dr. Rick Wilson of Oklahoma State University. The following simple scenario mimics the Black Book described in a Business Week article accessed February 2013)...
-
Step 1: Complete the following questionnaire. The following items ask you to assess how you experience your work setting. If you are not currently working, think back to a previous job, or...
-
Modify Solved Problem 10.4 to show that the PPF more closely approximates a quarter of a circle if there are five people rather than three. One of the two new people, Bill, can produce five piles of...
-
Assume that you are 35 years old, are married with two young children, are renting a condo, and have an annual income of $90,000. Use the following questions to guide your preparation of a rough...
-
For the following exercises, write the polynomial function that models the given situation. Consider the same rectangle of the preceding problem. Squares of 2x by 2x units are cut out of each corner....
-
Installment Repossession Entries selected transactions of TV Land Company are presented below. 1. A television set costing $540 is sold to Jack Matre on November 1, 2010, for $900. Matre makes a down...
-
Installment-Sales Computations and Schedules Saprano Company, on January 2, 2010, entered into a contract with a manufacturing company to purchase room-size air conditioners and to sell the units on...
-
Completed-Contract Method) Monat Construction Company, Inc., entered into a firm fixed price contract with Hyatt Clinic on July 1, 2010, to construct a four-story office building. At that time, Monat...
-
Cheyenne Company has the following information for the current period: Beginning inventory - $ 1 0 8 0 0 0 Purchases - $ 1 1 4 0 0 0 0 Ending Inventory - $ 1 1 8 0 0 0 Accounts Payable - $ 5 0 5 4 0...
-
You have just been hired by FAB Corporation, the manufacturer of a revolutionary new garage door opening device. The president asked you to review the company's costing system and "do what you can to...
-
Explain theories and models which examine organisational culture and human behaviour? You MUST provide an explanation of one theory or model which examines organisational culture., i.e. Schein,...

Study smarter with the SolutionInn App


