An alkane hydrocarbon has the formula C n H 2n+2 . The enthalpies of formation of the
Question:
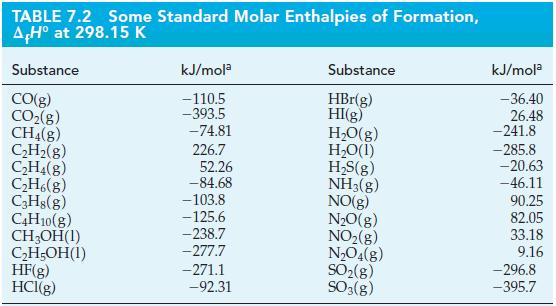
An alkane hydrocarbon has the formula CnH2n+2. The enthalpies of formation of the alkanes decrease (become more negative) as the number of C atoms increases. Starting with butane, for each additional CH2 group in the formula, ΔfH°, the enthalpy of formation, changes by about -21 kJ/mol. Use this fact and data from Table 7.2 to estimate the heat of combustion of heptane, C7H16(l).
Table 7.2
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, AH° at 298.15 K Substance CO(g) CO₂(g) CH4(g) C₂H₂(g) C₂H4(g) C₂H6(g) C3H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To estimate the heat of combustion of heptane C7H16 you can use the given information about the enthalpies of formation and the fact that the enthalpi...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Your friend says that the law of momentum conservation is violated when a ball rolls down a hill and gains momentum. What do you say?
-
What is the difference between a clock card and a time ticket?
-
A rigid beam \(B D\) is suspended from two rods \(A B\) and \(C D\) as shown in Fig. 13.29. Rod \(A B\) is of steel of \(20 \mathrm{~mm}\) diameter and rod \(B\) is of copper of \(25 \mathrm{~mm}\)...
-
Why are UC scenarios important to defining systems, product, and services?
-
Your supervisor, Jesse Baker, has asked you to begin working on data design tasks for the new information system, which will be implemented as a relational database. You will need to identify the...
-
Consider the following multiplication problem: Given an integer list (an array) of size n, we want to calculate the product of all numbers in the list and display the result. (a) Define an instance...
-
Martin Moreno is 42 years old, single, and works as a designer for a major architectural firm. He is well paid and over time has built up a sizable portfolio of investments. He considers himself an...
-
The metabolism of glucose, C 6 H 12 O 6 , yields CO 2 (g) and H 2 O(l) as products. Heat released in the process is converted to useful work with about 70% efficiency. Calculate the mass of glucose...
-
Some of the butane, C 4 H 10 (g), in a 200.0 L cylinder at 26.0 C is withdrawn and burned at a constant pressure in an excess of air. As a result, the pressure of the gas in the cylinder falls from...
-
(a) Should accounting transaction debits and credits be recorded directly in the ledger accounts? (b) What are the advantages of first recording transactions in the journal and then posting to the...
-
a) Consider a tiny economy of Country A which currently has a total output of $400 million, investment of $50 million and a multiplier of 2. The equilibrium equation for Fraser Island is represented...
-
Michael, a prior client, presents a Form W - 2 with more than one box 1 2 code. LaAdd up the amounts and report the total.Report only the value of the code from last year's tax return.Report only the...
-
The following table is entitled Advertising Spend and Revenues for Cola Companies in Country X (2022) and contains information on advertising spend and annual revenues for the top 5 cola companies in...
-
On April 30, 2024, Jarred Inc. issued 10,000 shares at $1.30 per share. After the share issuance, how many outstanding common shares would Jarred Inc. have and what would be Jarred Inc.'s common...
-
Franklin Company purchased a machine for leasing purposes on January 1, 2020, for $1,000,000. The machine has a 10-year life, has no residual value, and will be depreciated on a straight-line basis....
-
Safety in motels and hotels is a growing concern among travelers. Suppose a survey was conducted by the National Motel and Hotel Association to determine U.S. travelers perception of safety in...
-
Find the cross product a x b and verify that it is orthogonal to both a and b. a = (t, 1, 1/t), b = (t 2 , t 2 , 1)
-
What are the major characteristics of plant assets?
-
Mickelson Inc. owns land that it purchased on January 1, 2000, for $450,000. At December 31, 2010, its current value is $770,000 as determined by appraisal. At what amount should Mickelson report...
-
Name the items, in addition to the amount paid to the former owner or contractor, that may properly be included as part of the acquisition cost of the following plant assets. (a) Land. (b) Machinery...
-
Managerial Challenge: How exactly have computerization and information technology lowered costs at Chevron? Computerization and robotics have made output per worker higher and therefore lowered unit...
-
How do we calculate cross, and income elasticity for Chuck_Chicken? 1 Tear Hasth RibEQ (7) RibEP ChickP Disclac Trend 2 2003 12 63 $9.87 $1.85 9374.8 36 SUMMARY OUTPUT 3 2004 1 67 $9.92 $1.61 9435.9...
-
In our market-directed economy, producers provide goods and services that consumers demand as long as they can collect the selling price and can make a profit. However, there are some products and...

Study smarter with the SolutionInn App


