Describe a hybridization scheme for the central S atom in the molecule SF 4 that is consistent
Question:
Describe a hybridization scheme for the central S atom in the molecule SF4 that is consistent with the geometric shape pictured in Table 10.1. Which orbitals of the S atom are involved in overlaps, and which are occupied by lone-pair electrons?
Table 10.1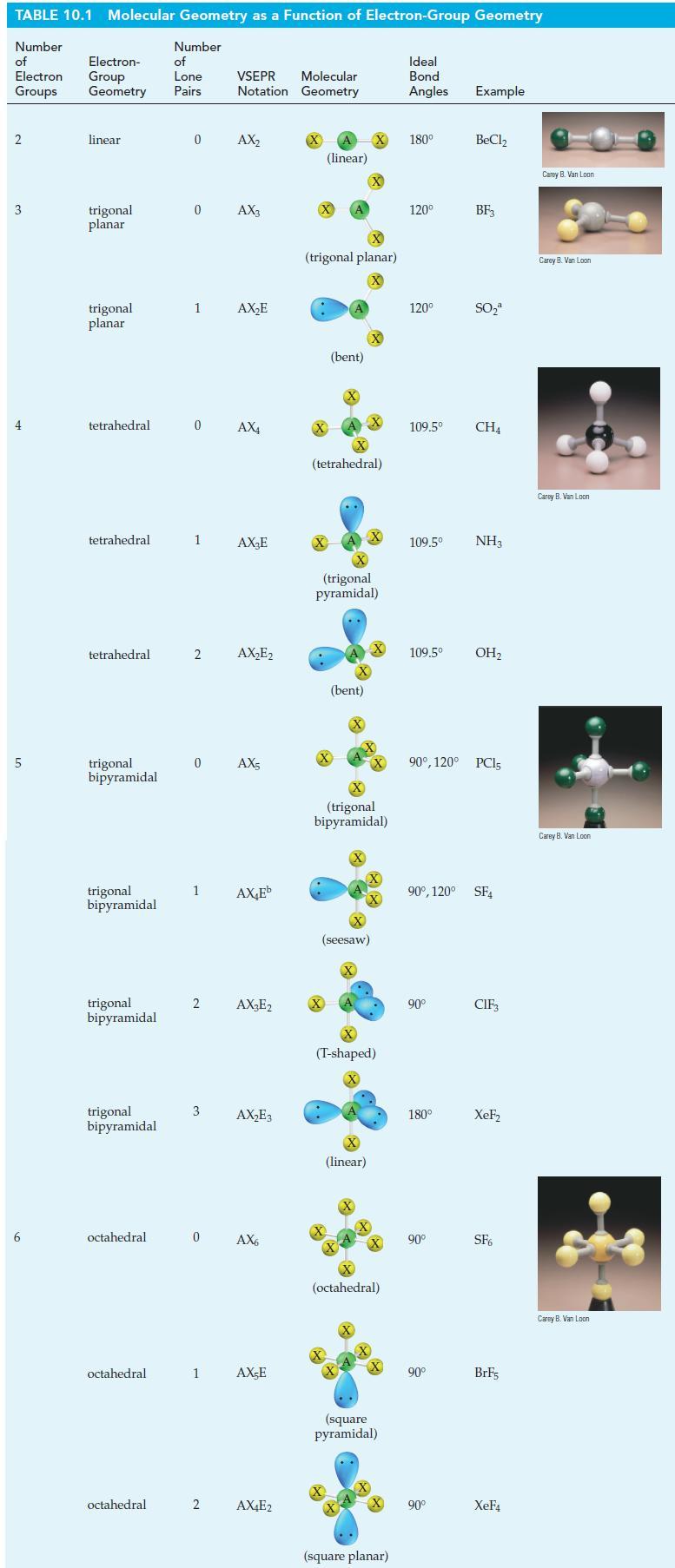
Transcribed Image Text:
TABLE 10.1 Molecular Geometry as a Function of Electron-Group Geometry Number of Electron Groups 2 3 4 5 6 Electron- Group Geometry linear trigonal planar trigonal planar tetrahedral tetrahedral tetrahedral trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal octahedral octahedral octahedral Number of Lone Pairs 0 0 1 1 2 0 AX4 1 2 3 Molecular VSEPR Notation Geometry 0 AX₂ AX3 0 AX5 2 AX₂E AX₂E AX₂E₂ AX₂Eb AX₂E2 AX₂E3 AX6 1 AX-E AX4E2 (linear) X (trigonal planar) X X (bent) (tetrahedral) .. X (trigonal pyramidal) (bent) X X (seesaw) X X (trigonal bipyramidal) X (linear) (T-shaped) X X +4+ (octahedral) X X (square pyramidal) (square planar) Ideal Bond Angles Example 180⁰ 120⁰ 120⁰ 109.5⁰ 109.5⁰ 109.5⁰ NH3 90° 180° BeCl₂ 90° BF3 90⁰, 120° PC15 90° SO₂ 90⁰, 120° SF4 90° CH4 OH₂ CIF₁ XeF₂ SF6 BrF5 XeF4 Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Describe a hybridization scheme for the central Cl atom in the molecule ClF 3 that is consistent with the geometric shape pictured in Table 10.1. Which orbitals of the Cl atom are involved in...
-
In ozone, O3, the two oxygen atoms on the ends of the molecule are equivalent to one another. (a) What is the best choice of hybridization scheme for the atoms of ozone? (b) For one of the resonance...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Consider the nutrition problem in Example 1 of Section 3.3. Solve the problem by the simplex method, and then determine the optimal quantities of soybeans and rice in the diet, and the new cost, if...
-
Amos orally agrees to hire Elizabeth for an eight-month trial period. Elizabeth performs the job magnificently, and after several weeks Amos orally offers Elizabeth a six-month extension at a salary...
-
An auditor is performing an attribute estimation sampling plan. Assuming a .05 acceptable risk of assessing control risk too low, a .04 tolerable rate of deviation, and a .01 expected population...
-
Art pervades almost every aspect of human social life. Give examples of art in the ways people make a living, in religion, in social structure, and in family life.
-
"In reviewing your previous reports, several questions came to Elizabeth Burkes mind. Use point and interval estimates to help answer these questions. 1. What proportion of customers rate the company...
-
What are the the differences between a Micro-manager and an Investor. Provide examples of each.
-
(A) Melamine is a carbonhydrogennitrogen compound used in the manufacture of adhesives, protective coatings, and textile finishing (such as in wrinkle-free, wash-and-wear fabrics). Its mass percent...
-
Propose a plausible Lewis structure, geometric structure, and hybridization scheme for the ONF molecule.
-
Refer to Question 3-24. Jaimes Hat Shop expects to incur the following expenses for each month of the second quarter of this financial year: In April, Jaimes had prepaid the rent for the whole year....
-
Create a paragraph or two for each question: 1. Reflect upon the manner in which social justice studies correspond and critically reflect on your understanding of the dynamics of privilege and...
-
In the accompanying diagram, PB and PD are secants drawn to circle O, PA = 8, PB = 20, and PD = 16. P A C 0 D What is PC? B
-
1. Understanding audience disposition will help you craft an effective message. How would you handle a situation if you misjudged your audience despite your best effort at audience analysis? Explain...
-
If you could exactly afford either 5 units of x and 21 units of y, or 9 units of x and 5 units of y, then if you spent all of your income on y, how many units of y could you buy?
-
Suppose that Tim is unable to buy the posters separately, but can buy the bundle of all five posters for a total price of $500. What is his consumer surplus?
-
Schatzberg Companys budget for its first year of operations follows. Fixed manufacturing cost .............. $150,000 Fixed selling and administrative costs .......... 200,000 Variable manufacturing...
-
For a Poisson process of rate , the Bernoulli arrival approximation assumes that in any very small interval of length , there is either 0 arrivals with probability 1- or 1 arrival with probability ....
-
Credit Instruments Describe each of the following: a. Sight draft b. Time draft. c. Bankers acceptance. d. Promissory note. e. Trade acceptance.
-
Trade Credit Forms in what form is trade credit most commonly offered? What is the credit instrument in this case?
-
Receivables Costs what costs are associated with carrying receivables? What costs are associated with not granting credit? What do we call the sum of the costs for different levels of receivables?
-
A Kite 50 feet above the ground moves horizontally at a speed of 8 ft/s. At what rate is the angle between the string and the ground decreasing when 150 feet of string is out? Preview My Answers...
-
Gravel is being dumped from a conveyor belt at a rate of 20 cubic feet per minute. It forms a pile in the shape of a right circular cone whose base diameter and height are always the same. How fast...
-
In 2 0 2 3 , Active Lifestyle Inc. purchased $ 4 , 3 6 4 , 0 0 0 of new plant & equipment, resulting in $ 4 , 3 6 4 , 0 0 0 in cash flows from investing activities. During the year, it raised $ 5 , 0...

Study smarter with the SolutionInn App


