The following r G values are given for 25 C. Combine the preceding equations, as necessary,
Question:
The following ΔrG° values are given for 25 °C.
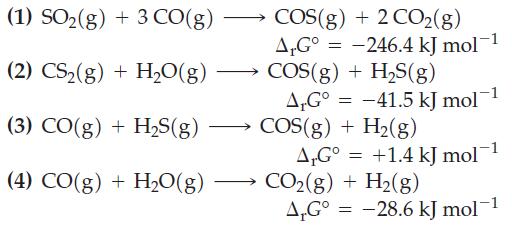
Combine the preceding equations, as necessary, to obtain ΔrG° values for the following reactions.
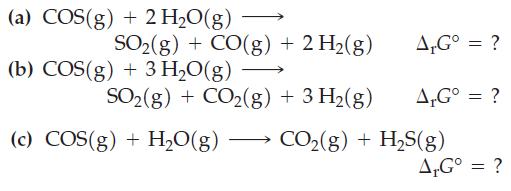
Of reactions (a), (b), and (c), which is spontaneous in the forward direction when reactants and products are present in their standard states?
Transcribed Image Text:
(1) SO₂(g) + 3 CO(g) (2) CS₂(g) +H₂O(g) (3) CO(g) + H₂S(g) (4) CO(g) + H₂O(g) COS(g) + 2 CO2(g) AG° = -246.4 kJ mol-1 COS(g) + H₂S(g) A,G° -41.5 kJ mol COS(g) + H₂(g) AG° +1.4 kJ mol-1 CO₂(g) + H₂(g) A.Gº = -28.6 kJ mol-¹ = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To obtain the ArG values for the given reactions you can sum the ArG values of the relevant reaction...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following r G values are given for 25 C. Combine the preceding equations, as necessary, to obtain r G values for each of the following reactions. Of reactions (a), (b), and (c), which would...
-
As the United States fell further and further into a recession in the years 2007-2009, the number of families who had to default on their mortgages and those who actually lost their homes grew....
-
A survey was conducted prior to the 2004 presidential election to explore the relationship between a persons religious fervor and their choice of a political candidate. Voters were asked how often...
-
Find an equation of the tangent plane at the given point. f(x, y) = x + y, (4,1)
-
What costs are excluded from the cost base when absorption-cost pricing is used to determine the markup percentage?
-
How does the riskiness of this 2-stock portfolio compare with the riskiness of the individual stocks if they were held in isolation?
-
Barbara Vigil, Chief Justice, New Mexico Supreme Court Ken Badilla bought a pair of Brahma brand work boots from Wal-Mart on October 19, 2003. The boots packaging had these express descriptions: iron...
-
The chart of accounts of Lopez Company includes the following selected accounts. 112 Accounts Receivable 401 Sales 120 Merchandise Inventory 412 Sales Returns and Allowances 126 Supplies 505 Cost of...
-
The money supply process involves various factors, including the actions of commercial banks, the central bank's monetary policy, and the public's demand for money. My questions are: How do these...
-
Together with the following data, to estimate the bond-dissociation energy of the F 2 molecule. Compare your result with the value listed in Table 10.3. Table 10.3 F(g) - 2 F(g) A.G = 123.9 kJ mol-1
-
At 298 K, for the reaction 2 PCl 3 (g) + O 2 (g) 2 POCl 3 (l), r H = -620.2 kJ mol -1 and the standard molar entropies, in J mol 1 K 1 , are PCl 3 (g), 311.8; O 2 (g), 205.1; and POCl 3 (l), 222.4....
-
If you worked in the advertising department of a premium-priced furniture manufacturer, would you recommend magazine advertising? Why or why not?
-
What are the characteristics of a multi-domestic corporation?
-
Which of the approaches to going green (see Exhibit 6-3) does Starbucks utilize? Explain your choice. High Low Environmental Sensitivity Activist Approach (Dark Green) Stakeholder Approach Market...
-
What do you think of Starbuckss goal to stop using plastic straws worldwide by 2020? What challenges might it face in meeting that goal? Is this merely a public relations promotion?
-
Which decision style best describes Kevin Johnsons approach to decision making? Which decision style best summarizes Howard Schultzs decision-making approach? Explain your answer.
-
What are specific ways in which Starbucks has shown top-management commitment to diversity? In what ways could Starbucks become even stronger in the area of diversity?
-
Federated Banks is interested in consumer-oriented sales promotions that would encourage senior citizens to direct deposit their Social Security checks with the bank. Evaluate the sales promotion...
-
Write the statement to store the contents of the txtAge control in an Integer variable named intAge.
-
High-low method Ken Howard, financial analyst at JVR Corporation, is examining the behavior of quarterly maintenance costs for budgeting purposes. Howard collects the following data on machine-hours...
-
High-low method and regression analysis. Happy Business College has recently opened a restaurant as part of its hospitality major. For the first 10 weeks the manager did not estimate any costs, but...
-
High-low method, regression analysis. Anna Martinez, the financial manager at the Casa Real restaurant is checking to see if there is any relationship between newspaper advertising and sales revenues...
-
10. Develop the truth table for each of the combinational logic circuits shown below. b.) A B C D X
-
10. Develop the truth table for each of the combinational logic circuits shown below. a.) AB C Do X
-
3. Create a div element with a width and height of 500px. Create a radial gradient with three colors. Start the gradient in the bottom-left corner with the colors changing as Page 1 of 2 they move...

Study smarter with the SolutionInn App


