We have noted that an emission spectrum is a kind of atomic fingerprint. The various steels are
Question:
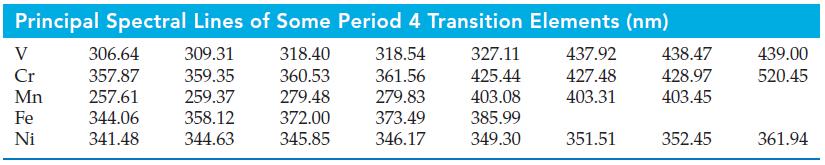
We have noted that an emission spectrum is a kind of “atomic fingerprint.” The various steels are alloys of iron and carbon, usually containing one or more other metals. Based on the principal lines of their atomic spectra, which of the metals in the table above are likely to be present in a steel sample whose hypothetical emission spectrum is pictured? Is it likely that still other metals are present in the sample? Explain.
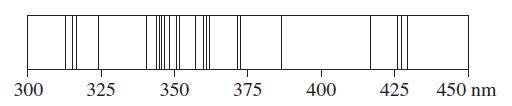
▲ Hypothetical emission spectrum
In a real spectrum, the photographic images of the spectral lines would differ in depth and thickness depending on the strengths of the emissions producing them. Some of the spectral lines would not be seen because of their faintness.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





