Balmer seems to have deduced his formula for the visible spectrum of hydrogen just by manipulating numbers.
Question:
Balmer seems to have deduced his formula for the visible spectrum of hydrogen just by manipulating numbers. A more common scientific procedure is to graph experimental data and then find a mathematical equation to describe the graph. Show that equation (8.4) describes a straight line. Indicate which variables must be plotted, and determine the numerical values of the slope and intercept of this line. Use data from Figure 8-12 to confirm that the four lines in the visible spectrum of hydrogen fall on the straight-line graph.
Eq. 8.4
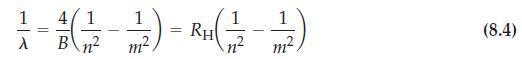
Figure 8-12
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:




