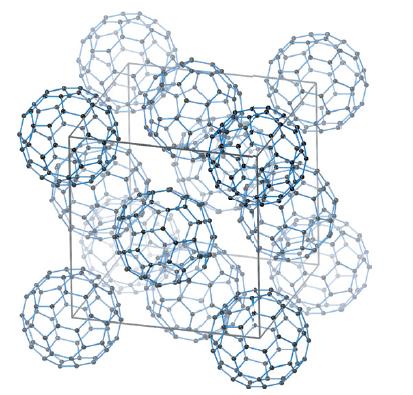
(a) Potassium reacts with C 60 (Fig. 4.16) to give a compound in which all the octahedral...
Question:
(a) Potassium reacts with C60 (Fig. 4.16) to give a compound in which all the octahedral and tetrahedral holes are filled by potassium ions. Derive a stoichiometry for this compound.
(b) The reaction of C60 with excess potassium yields a material that has a body-centred arrangement of C60 molecules with two potassium ions along each unit cell edge; calculate the stoichiometry of this compound.
Figure 4.16.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





