(a) Suggest how Na will react with MeC(CH 2 SbCl 2 ) 3 . (b) Comment on...
Question:
(a) Suggest how Na will react with MeC(CH2SbCl2)3.
(b) Comment on aspects of the bonding in the following compound:
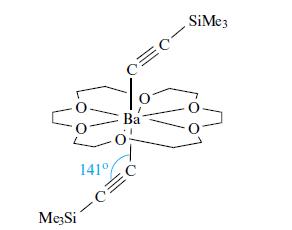
(c) Cp2Ba and (C5Me5)2Ba both have polymeric structures in the solid state. However, whereas Cp2Ba is insoluble in common organic solvents, (C5Me5)2Ba is soluble in aromatic solvents. In contrast to (C5Me5)2Ba, (C5Me5)2Be is monomeric. Suggest a reason for these observations.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





