Both HBr and BrO (see problem 17.23) are present in volcanic plumes. A model for reactions in
Question:
Both HBr and BrO (see problem 17.23) are present in volcanic plumes. A model for reactions in the plume involves the following sequence:
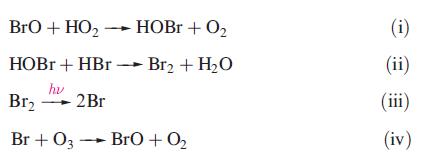
(a) Briefly discuss the types of reactions shown above.
(b) Discuss the roles of ClO and Cl in ozone depletion over Antarctica. Comment on any similarities between these reactions and those occurring in the volcanic plume.
Data from Problem 17.23
BrO has been detected in the emission gases from volcanoes (N. Bobrowski et al. (2003) Nature, vol. 423, p. 273). Construct an MO diagram for the formation of BrO from Br and O atoms. Comment on any properties and bonding features of BrO that you can deduce from the diagram.
[Cl2O2]+ is approximately planar and is described as a charge transfer complex of [Cl2]+ and O2. By considering the HOMOs and LUMOs of [Cl2]+ and O2, suggest what orbital interactions are involved in the charge transfer.
Step by Step Answer:






