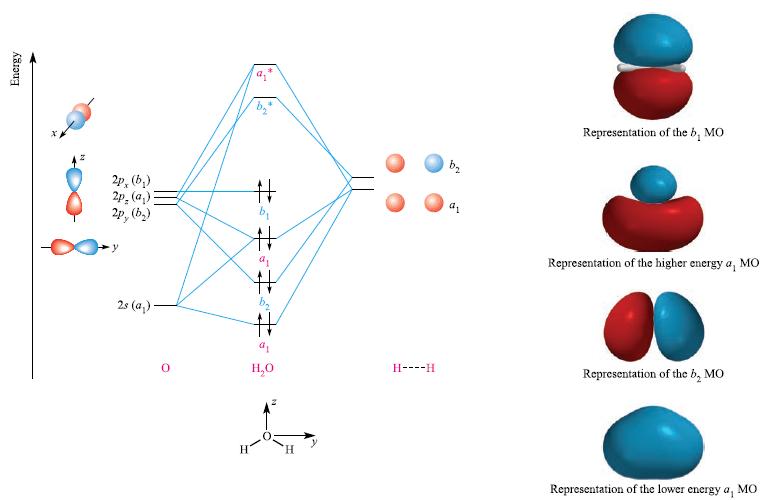
Table 5.6 gives the results of a self-consistent field (SCF) quantum chemical calculation for H 2 O
Question:
Table 5.6 gives the results of a self-consistent field (SCF) quantum chemical calculation for H2O using an orbital basis set of the atomic orbitals of O and the LGOs of an H---H fragment. The axis set is as defined in Fig. 5.15.
(a) Use the data to construct pictorial representations of the MOs of H2O and confirm that Fig. 5.15 is consistent with the results of the calculation.
(b) How does MO theory account for the presence of lone pairs in H2O?
Table 5.6
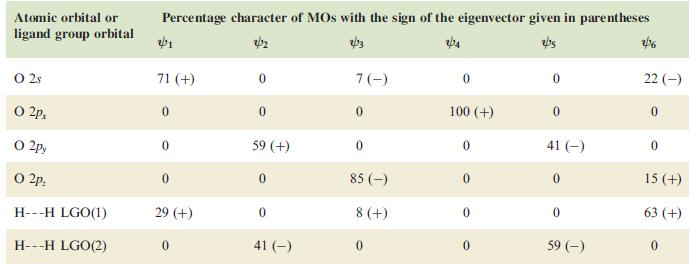
Figure 5.15
Transcribed Image Text:
Atomic orbital or ligand group orbital 0 2s 0 2px O 2py O 2p: H---H LGO(1) H---H LGO(2) Percentage character of MOs with the sign of the eigenvector given in parentheses 24 71 (+) 0 0 0 29 (+) 0 0 0 59 (+) 0 0 41 (-) 7(-) 0 0 85 (-) 8 (+) 0 0 100 (+) 0 0 0 0 0 41 (-) 0 0 59 (-) 2/6 22 (-) 0 0 15 (+) 63 (+)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
To construct pictorial representations of the molecular orbitals MOs of H2O using the data from Table 56 we first need to identify the relevant data f...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the sample results in Exercise 11.14, construct and interpret the 95% confidence interval for the difference between the population means. Is the hypothesized difference (0.00) within the...
-
The article "Effects of Household Fabric Softeners on Thermal Comfort of Cotton and Polyester Fabrics After Repeated Launderings" (Family and Consumer Science Research J., 2009: 535-549) reported the...
-
In this exercise we consider how to deal with class lengths that are unequal (and with open-ended classes) when setting up histograms. Often data are published in this form and we wish to construct a...
-
What is the "ALDI Way" and what was its quest? Take out costs; eliminate complexity Survival; make enough money to pay overhead costs Increase market share; find more prospective customers Build...
-
To remain competitive, a steel company needs to reconfigure its operations to align with worldwide production. As a consultant on world steel production, provide a report that indicates appropriate...
-
Explain expert networks. How can expert networks affect the trading of specific stocks?
-
The Nielsen family formed their corporation, N. Robert Nielsen, Inc., to conduct farming operations. Morre, Grider & Co. is a certified public accounting firm that has provided accounting, tax, and...
-
A perishable dairy product is ordered daily at a particular supermarket. The product, which costs $1.19 per unit, sells for $1.65 per unit. If units are unsold at the end of the day, the supplier...
-
1 Problem 4 - A firm's optimal output choice (10 points) Consider a price taking firm, where the market price for the output is given by P. The firm's output choice is denote by Q. The firm has a...
-
Refer to Fig. 5.17 and the accompanying discussion. (a) Why does the B 2p z atomic orbital become a non-bonding MO in BH 3 ? (b) Draw schematic representations of each bonding and antibonding MO in...
-
Using Figs. 5.22, 5.23 and 5.25 to help you, compare the MO pictures of the bonding in BF 3 and [NO 3 ]. What approximations have you made in your bonding analyses? Figure 5.22. Figure 5.23 Figure...
-
Maria purchased 1,000 shares of stock for $35.50 per share in 2003. She sold them in 2007 for $55.10 per share. Express her capital gain as a percent, rounded to the nearest tenth of a percent
-
How does working capital impact a firms value?
-
Company A recorded a profit before tax of $2,500,000 for the year ended 31 December 20x3. The tax rate for 20x3 was 24% while that of 20x2 was 22%. Deferred tax liability as at 31 December 20x2 was...
-
What is the difference between direct paper and dealer paper?
-
What is the difference between temporary and permanent working capital?
-
What mechanisms allow hostile acquirers to get around the free rider problem in takeovers?
-
Describe a periodic inventory system. Identify a type of company that might use it.
-
The trade-off theory relies on the threat of financial distress. But why should a public corporation ever have to land in financial distress? According to the theory, the firm should operate at the...
-
Why is the platinum/rhodium catalyst in automobile catalytic converters dispersed on the surface of a ceramic rather than used in the form of a thin metal foil?
-
Which inorganic materials, other than graphite, have structures with strong in-layer bonds but much weaker interactions between layers, and so might be exfoliated into single sheets?
-
(a) Draw a schematic diagram of a coreshell nanoparticle. (b) Briefly describe how coreshell nanoparticles could be made using either vapour-phase or solution-based techniques. (c) For what purpose...
-
An instrument, maintained at 118.7 C, has two separate flasks that contain different gases. If the flasks are opened so that the gases are able to mix, what is the final density in flask 2? Flask Gas...
-
Compute the discount on a 10 year simple discount loan if the amount borrowed is $1,190 and the annual simple discount rate is 4.4%.
-
g. Switch to Datasheet View then choose Investment Banking & Asset Management for the Industry field for CompanyID 2 (Des Moines Financial Group).

Study smarter with the SolutionInn App


