The catalyst [Rh(Ph 2 PCH 2 CH 2 PPh 2 )] + can be prepared by the
Question:
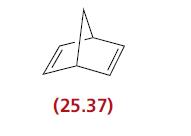
The catalyst [Rh(Ph2PCH2CH2PPh2)]+ can be prepared by the reaction of [Rh(nbd)(Ph2PCH2CH2PPh2)]+ (nbd = 25.37) with two equivalents of H2. In coordinating solvents [Rh(Ph2PCH2CH2PPh2)]+, in the form of a solvated complex [Rh(Ph2PCH2CH2PPh2)(solv)2]+, catalyses the hydrogenation of RCH=CH2.
(a) Draw the structure of [Rh(nbd)(Ph2PCH2CH2PPh2)]+ and suggest what happens when this complex reacts with H2.
(b) Draw the structure of [Rh(Ph2PCH2CH2PPh2)(solv)2]+, paying attention to the expected coordination environment of the Rh atom.
(c) Given that the first step in the mechanism is the substitution of one solvent molecule for the alkene, draw a catalytic cycle that accounts for the conversion of RCH=CH2 to RCH2CH3. Include a structure for each intermediate complex and give the electron count at the Rh centre in each complex.
Step by Step Answer:






