The thermite process is shown in eq. 13.5. Determine r H for this reaction if
Question:
The thermite process is shown in eq. 13.5. Determine ΔrH° for this reaction if ΔfH°(Al2O3, s, 298K) and ΔfH°(Fe2O3, s, 298K) = −1675:7 and −824.2 kJ mol−1, and comment on the relevance of this value to that of ΔfusH(Fe; s) = 13:8 kJ mol−1.
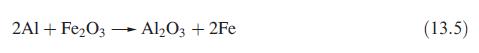
Transcribed Image Text:
2A1+ Fe₂O3 Al₂O3 + 2Fe (13.5)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
To determine rH the standard molar enthalpy of reaction for the thermite process shown in equation 135 we can use the following equation rH fHproducts ...View the full answer

Answered By

Krishnavendra Y
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagiarism), well-researched and critically analyzed papers.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Solid oxide fuel cells (SOFC) have been proposed as an alternative energy technology for use in large stationary power applications (1 to 10MWof electrical power). These devices have an ion...
-
Suppose we want to make change for 10 cents, using the least number of coins of denominations 1, 3e, 4e and 7e. Apply a dynamic programming algorithm to get an optimum solution for the above problem...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Ebbers Corporation overstated its ending inventory balance by $15,000 in the current year. What impact will this error have on cost of goods sold and gross profit in the current year and following...
-
List and briefly explain four assumptions underlying discounted-cash-flow analysis.
-
How many predictors are in the model? Use information in the ANOVA table below, which comes from fitting a multiple regression model to predict the prices for horses (in $1000s). Source Regression...
-
Management is considering three alternatives to satisfy an urgent need. Each of the alternatives will completely satisfy the need, so no combinations have to be considered. The first costs, operating...
-
Costello Advertising Agency Inc. was founded by Pat Costello in January of 2013. Presented on the next page are both the adjusted and unadjusted trial balances as of December 31, 2014. Instructions...
-
1/ You have two different assets (investments). Asset A (perpetuity) will pay you $1,000 in one year, $1,000 in two years, $1,000 in three years, and so on every year forever. Asset B will pay you...
-
(a) One gallium-containing product, A, was obtained from the following reaction, carried out in Et 2 O solvent: The room temperature, solution 1 H NMR spectrum of A showed the following signals: ...
-
Plot a graph to show the variation in values of IE 1 , IE 2 and IE 3 for the group 13 elements (Table 13.1), and plot a similar graph to show the variation in values of IE 1 and IE 2 for the group 2...
-
A regional manager visits local fast-food franchises and evaluates the speed of the service. If the manager receives her meal within 45 seconds, the server is given a free movie-admission coupon. If...
-
What factors determine the appropriate authority and control structure in (a) a research and development laboratory, (b) a large department store, and (c) a small manufacturing company?
-
What makes some tasks more complex than others? Give an example of an organization that uses each of the four types of technology identified by Perrow.
-
In what ways can the informal organization and the norms and values of its culture affect the shape of an organization?
-
As organizations grow and differentiate, what problems can arise with a functional structure?
-
In what ways can organizational culture increase organizational effectiveness? Why is it important to obtain the right fit between organizational structure and culture?
-
Campbell Ltd. invested in a joint venture by providing cash of $160,000. Campbell obtained a 22% interest in the joint venture based on its contribution. During the year, the joint venture earned...
-
The area of a rectangle is 30 cm 2 and its perimeter is 26 cm. Find the length and width of the rectangle.
-
Determine the composition of a compound based on the ReO 3 structure, Exercise 4.17, where each of the oxide ions is replaced by a hydroxide ion [OH] . Predict, based on commonly adopted oxidation...
-
The ReO 3 structure is cubic with a rhenium atom at each corner of the unit cell and one oxygen atom on each unit cell edge midway between the rhenium atoms. Sketch this unit cell and determine (a)...
-
Consider the structure of caesium chloride. How many Cs + ions occupy second-nearest-neighbour locations of a Cs + ion? How many Cl ions occupy the third-nearest-neighbour sites?
-
this task you must find a community group that you can work with to research community priorities as well as participate in some work relating to the group. There must be at least three individuals...
-
what ways does the study of the extended phenotype, encompassing the ecological effects, behaviors, and artifacts produced by organisms, expand our understanding of genotype-phenotype-environment...
-
A project requires an inital nvested of 37,000 dollars. The instream cashflow is 10000 dollars per year. The time value of money is 4.52 percent. Compute the payback period Add your answer

Study smarter with the SolutionInn App


