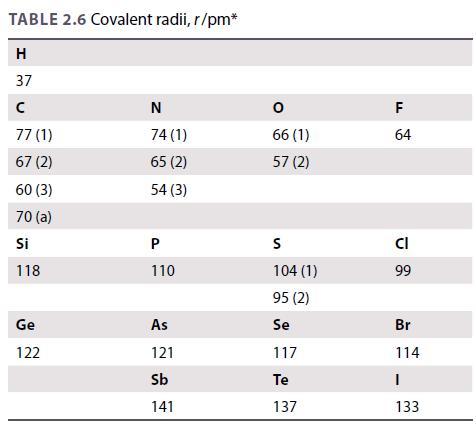
Use the covalent radii in Table 2.6 to calculate the bond lengths in (a) CCl 4 (177
Question:
Use the covalent radii in Table 2.6 to calculate the bond lengths in
(a) CCl4 (177 pm),
(b) SiCl4 (201 pm),
(c) GeCl4 (210 pm).
(The values in parentheses are experimental bond lengths and are included for comparison.)
Table 2.6.
Transcribed Image Text:
TABLE 2.6 Covalent radii, r/pm* H 37 с 77 (1) 67 (2) 60 (3) 70 (a) Si 118 Ge 122 N 74 (1) 65 (2) 54 (3) P 110 As 121 Sb 141 0 66 (1) 57 (2) S 104 (1) 95 (2) Se 117 Te 137 F 64 CI 99 Br 114 I 133
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To calculate the bond lengths in the given molecules CCl4 SiCl4 and GeCl4 using the covalent radii i...View the full answer

Answered By

Abdul Wahab Qaiser
Before working at Mariakani, I volunteered at a local community center, where I tutored students from diverse backgrounds. I helped them improve their academic performance and develop self-esteem and confidence. I used creative teaching methods, such as role-playing and group discussions, to make the learning experience more engaging and enjoyable.
In addition, I have conducted workshops and training sessions for educators and mental health professionals on various topics related to counseling and psychology. I have presented research papers at conferences and published articles in academic journals.
Overall, I am passionate about sharing my knowledge and helping others achieve their goals. I believe that tutoring is an excellent way to make a positive impact on people's lives, and I am committed to providing high-quality, personalized instruction to my students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
Calculate the CH and CCl bond lengths in chloroform, CHCl3, using values for the covalent radii from Table 9.4. How do these values compare with the experimental values: CH, 107 pm; CCl, 177 pm?...
-
Calculate the CCl and CC bond lengths in ethyl chloride, C 2 H 5 Cl, using values for the covalent radii from Table 9.4. How do these values compare with the experimental values: CCl, 177; CC, 155...
-
Calculate the bond length for each of the following single bonds, using covalent radii (Table 9.4): a. CH b. SCl c. BrCl d. SiO TABLE 9.4 Single-Bond Covalent Radii for Nonmetallic Elements (in pm)...
-
Number of toys produced 60,000 120,000 150,000 O $0.20 What is the materials cost per unit of output? O $0.30 Starfun Toys, Inc. Cost of Materials O $0.70 O $0.50 Total cost of materials $18,000...
-
You want to have $2 million in real dollars in an account when you retire in 40 years. The nominal return on your investment is 10 percent and the inflation rate is 3.8 percent. What real amount must...
-
In problem, convert each angle in degrees to radians. Express your answer in decimal form, rounded to two decimal places. 73
-
Defendants Jack and Claire Lein owned and lived on Willow Creek Farm from 1980 through 2004. The farm manager, Stewart, and his girlfriend, plaintiff Tambra Curtis, also lived on the farm during this...
-
The following transactions of Emergency Pharmacies occurred during 2014 and 2015: 2014 Mar 1 Borrowed $360,000 from Lessburg Bank. The six-year, 10% note requires payments due annually, on March 1....
-
(5) Strong Data Processing Inequality for the Divergence. Given finite alphabets X and Y, consider a conditional probability distribution W(y|x), for (x, y) Xxy, such that for some y. y, W (y, x) > c...
-
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2 (g) + O 2 (g) 2 H 2 O(g). The experimental value is 484 kJ. Account for the difference between the estimated and...
-
What shapes would you expect for the species (a) ClF 3 , (b) lCl 4 , (c) I 3 ?
-
Why do companies implement competitive pricing strategies?
-
What are the strategical elements of logistics in business? Explain with example
-
ABC, Inc., has a beta of 2.03. The risk-free rate is 2.3% and the market risk premium is 6%. What is the required rate of return on ABC's stock? Note: Convert your answer to percentage and round off...
-
In 2023, Anna and Matt earn $420,000 of adjusted gross income and figure $50,000 of total itemized deductions on their Schedule A, comprised entirely of qualified home mortgage interest and gifts to...
-
You are analyzing the stock of Pizza Hub, an online pizza delivery company. Your estimate of the cost of equity is 16 percent. The most recent dividend is $0.20 per share. You expect dividends to...
-
Do organizations such as Apple aspire to cultivate a high inventory turnover ratio to enhance sales momentum while simultaneously mitigating inventory expenses?
-
A pharmaceutical company is considering investing in a new and improved vitamin D supplement for children. Vitamin D, whether ingested as a dietary supplement or produced naturally when sunlight...
-
Distinguish between the work performed by public accountants and the work performed by accountants in commerce and industry and in not-for-profit organisations.
-
Consider the following two scenarios: (i) Aluminium rivets used to connect two steel plates, (ii) Steel rivets used to connect two Aluminium plates. Discuss whether these choices would be sensible.
-
The commercial purification of copper metal is carried out in electrolytic cells. The anode is composed of impure (blister) copper, and the electrolyte is a mixture of aqueous CuSO 4 and H 2 SO 4 ....
-
In each of the following reactions, relate starting materials and products by the processes of reduction, oxidation, disproportionation or no redox change. In some reactions, more than one process is...
-
The displacement of a particle is given by s = 2t-39t + 95t-47 where s is in feet and t is in seconds. Plot the displacement, velocity, and acceleration as functions of time for the first 15 seconds...
-
5. A piston/cylinder contains 1.5 kg of air at 300 K and 160 kPa. It is now heated to 900 K. There is a linear spring mounted on the piston such that when the air is heated, the final volume is twice...
-
6. Calculate the pressure at B, C, D, N, and E with the flow rate Q=0.56ft/s 1-OFE 2in/2in When du hose diameter JAN = Nozda 1.8 Ft /// (41) REFERENCE pant Where d Nozzle Diameter, 0.8 in N= dhorse...

Study smarter with the SolutionInn App


