Students and faculty from Brigham Young University developed and delivered a small-scale process to Tonga for making
Question:
Students and faculty from Brigham Young University developed and delivered a small-scale process to Tonga for making biodiesel fuel from coconut oil. The central component of the process was the reactor where the coconut oil (triglycerides containing a variety of fatty acids - represented as R1, R2, and R3) is reacted with methanol in the presence of sodium hydroxide (acting as a catalyst, thus not consumed) as shown in the following reaction:
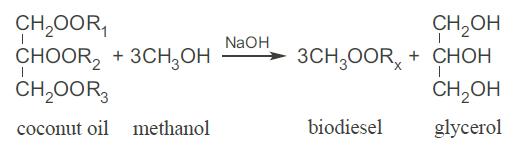
The conversion of coconut oil is essentially 100%, and the products are biodiesel, which is a collection of fatty esters (the collection of several varieties are represented here by the designation Rx), and glycerol.
For the small-scale process (assume steady-state), 140 L/hr of coconut oil is fed to the reactor, along with 540 g/hr solid (pure) NaOH. Based on previous testing, methanol is also fed at a molar flow rate equal to 4.2 times the molar flow rate of coconut oil. A single outlet stream leaves the reactor. The properties of all components are as follows:
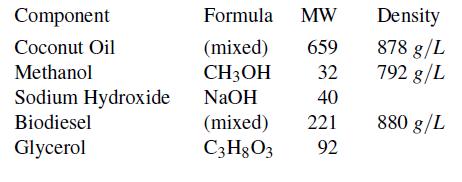
a. What is the mass fraction of biodiesel in the reactor output?
b. Later stages of the process separate the components of this reactor output to provide a stream of essentially pure biodiesel. What is the volumetric flow rate of that stream?
Step by Step Answer:

Introduction To Chemical Engineering Tools For Today And Tomorrow
ISBN: 9780470885727
5th Edition
Authors: Kenneth A. Solen, John N. Harb





