P-V-T behavior of a simple fluid is found to obey the equation of state given in problem
Question:
P-V-T behavior of a simple fluid is found to obey the equation of state given in problem 8.14.
(a) Derive a formula for the enthalpy departure for the fluid.
(b) Determine the enthalpy departure at 20 bar and 300 K.
(c) What value does the entropy departure have at 20 bar and 300 K?
Data from Problem 8.14:
A 1 m3 isolated chamber with rigid walls is divided into two compartments of equal volume. The partition permits transfer of heat. One side contains a nonideal gas at 5 MPa and 300 K and the other side contains a perfect vacuum. The partition is ruptured, and after sufficient time for the system to reach equilibrium, the temperature and pressure are uniform throughout the system. The objective of the problem statements below is to find the final T and P.
The gas follows the equation of state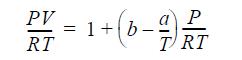
where b = 20 cm3/ mole; a = 40,000 cm3K/mole; and CP = 41.84 0.084T(K) J/mol-K.
(a) Set up and simplify the energy balance and entropy balance for this problem.
(b) Derive formulas for the departure functions required to solve the problem.
(c) Determine the final P and T.
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





