A vaporliquid phase diagram for a binary mixture of species 1 and 2 at 293 K is
Question:
A vapor–liquid phase diagram for a binary mixture of species 1 and 2 at 293 K is shown in the following fi gure.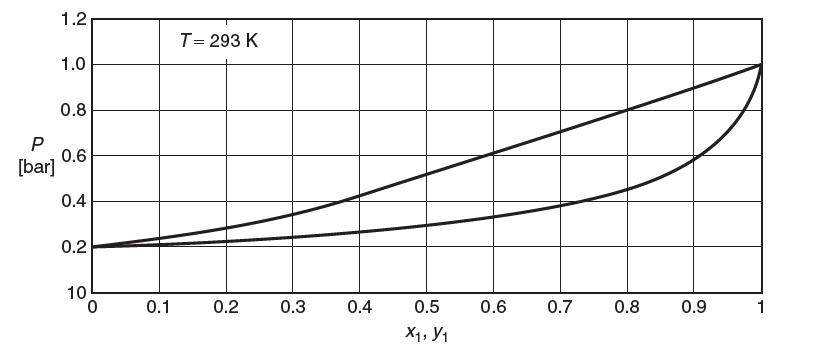
Answer the following questions.
(a) At 293 K, what is the value of P1sat?
(b) Consider a mixture of 0.2 mol species 1 and 0.8 mol species 2 at 0.4 bar and 293 K.
(i) What phase or phases are present?
(ii) What is the composition of each phase?
(iii) How many moles are in each phase?
(c) Consider a mixture of 1.2 mol species 1 and 0.8 mol species 2 at 0.4 bar and 293 K.
(i) What phase or phases are present?
(ii) What is the composition of each phase?
(iii) How many moles are in each phase?
(d) Estimate the value of the two-suffi x Margules parameter, A.
(e) Consider the steady-state fl ash operation depicted here: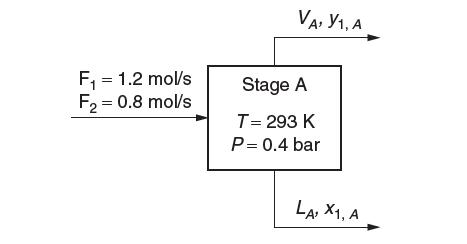
What are the values for the fl ow rate, LA, and composition, xA, of the exit liquid stream and the fl ow rate, VA, and composition, yA, of the exit vapor streams?
(f) Consider the two-stage steady-state fl ash operation depicted here: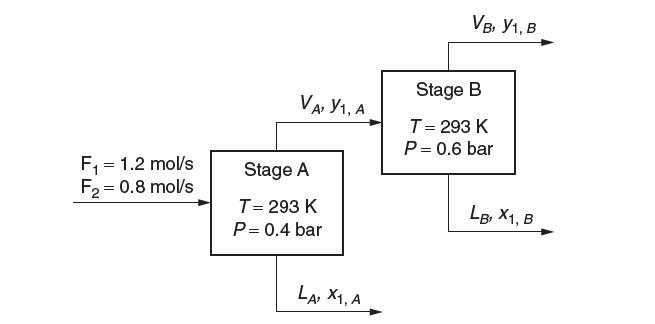
What are the values for the fl ow rates and compositions of the exit liquid and vapor streams?
Step by Step Answer:






