On a hot summer day, you want to cool two glasses of warm lemonade, but have no
Question:
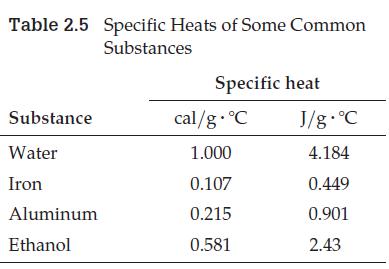
On a hot summer day, you want to cool two glasses of warm lemonade, but have no ice. Not wanting to wait until you can make some, you place two small metal blocks in the freezer. One block is pure iron, the other pure aluminum, and each has a mass of exactly 50 g. After both have cooled to –10°C, you put them into separate glasses and add 200 mL of warm lemonade to each. After a few minutes, both blocks have warmed up to +10°C. At this point, is the lemonade in one glass cooler than the lemonade in the other glass? If so, which is cooler and why? (Despite all the numerical information, you should be able to use specific heat values from Table 2.5 to answer without doing any calculations.)
Step by Step Answer:

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver





