Repeat the analysis in Problem 2.3 for either one of the terminal CC bonds of butane. Problem
Question:
Repeat the analysis in Problem 2.3 for either one of the terminal C—C bonds of butane.
Problem 2.3
(a) Draw a Newman projection for each staggered and eclipsed conformation about the C2–C3 bond of isopentane, a compound containing a branched carbon chain.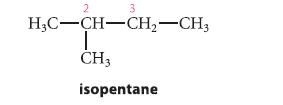
Show all staggered and eclipsed conformations.
(b) Sketch a curve of potential energy versus dihedral angle for isopentane, similar to that of butane in Fig. 2.5. Label each energy maximum and minimum with one of the conformations you drew in part (a).
(c) Which of the conformations you drew in part (a) are likely to be present in greatest amount in a sample of isopentane?
Explain.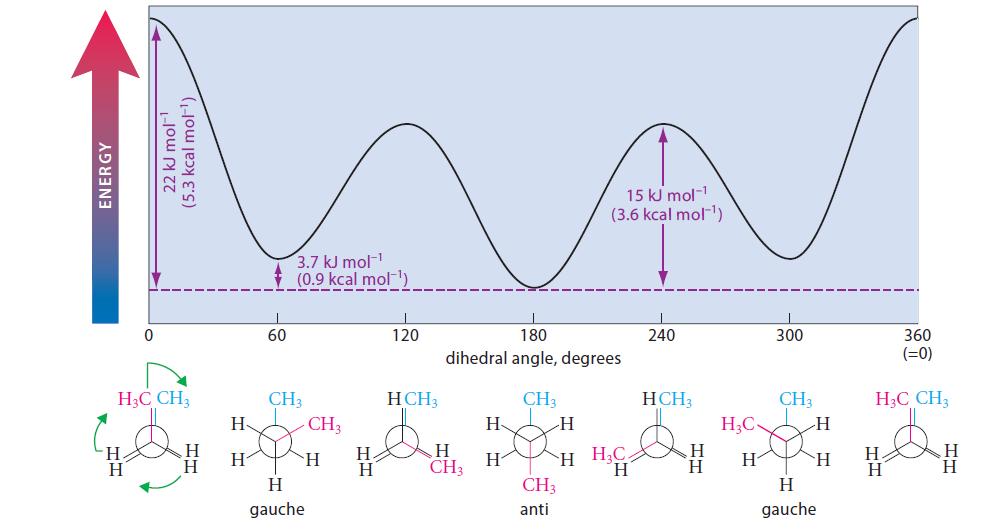
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





