The reaction given in Fig. P17.59 occurs by a mechanism called the S N 2 mechanism, which
Question:
The reaction given in Fig. P17.59 occurs by a mechanism called the SN2′ mechanism, which is a bimolecular substitution that occurs by reaction of the nucleophile at an allylic carbon. In this reaction, the C—Cl bond as well as the bond to the nucleophile must be perpendicular to the plane of the alkene double bond for proper orbital overlap, but this orientation can occur in two ways: The C—Cl bond can be syn to the new bond that is formed with the nucleophile, or it can be anti. Is the following SN2′ reaction syn or anti? How does this result contrast with the stereochemistry of the SN2 reaction?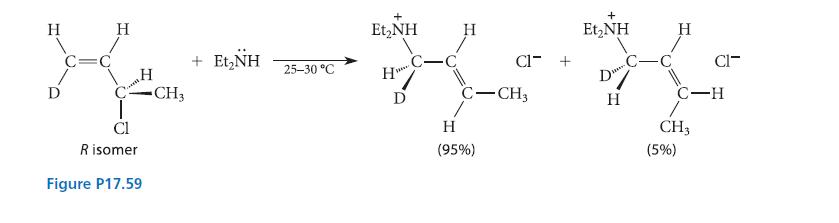
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





