When S-cyclohexyl thioacetate is reduced by LiAlH 4 in ether, followed by protonolysis, cyclohexanethiol is formed. However,
Question:
When S-cyclohexyl thioacetate is reduced by LiAlH4 in ether, followed by protonolysis, cyclohexanethiol is formed. However, when a large excess of the Lewis acid BF3 is added to the reaction mixture before the reduction, cyclohexyl ethyl sulfide is formed (Fig. P25.26). Account mechanistically for the effect of BF3 in changing the outcome of the reaction.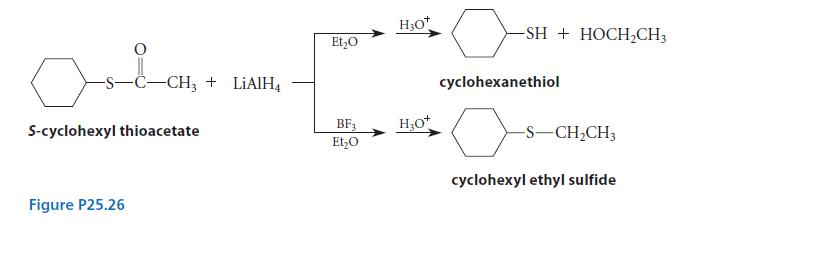
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





