Among the best-known building blocks in nanotechnology applications are nanoparticles of noble metals. For example, colloidal suspensions
Question:
Among the best-known building blocks in nanotechnology applications are nanoparticles of noble metals. For example, colloidal suspensions of silver or gold nanoparticles (10–200 nm) exhibit vivid colors because of intense optical absorption in the visible spectrum, making them useful in colorimetric sensors.
In the illustration shown below, a suspension of gold nanoparticles of a fairly uniform size in water exhibits peak absorption near a wavelength of 525 nm (near the blue region of the visible spectrum of light). When one views the solution in ambient (white) light, the solution appears wine-red because the blue part of the spectrum is largely absorbed. When the nanoparticles aggregate to form large particles, an optical absorption peak near 600–700 nm (near the red region of the visible spectrum) is observed. The breadth of the peak reflects a fairly broad particle size distribution. The solution appears bluish because the unabsorbed light reaching the eye is dominated by the short (blue-violet) wavelength region of the spectrum.
![0.7 0.6 0.4 0.2 350 525 600 800 1000 Wavelength [nm] Abs](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1590/0/5/9/5345ec6620e7b4f21590059528903.jpg)
Since the optical properties of metallic nanoparticles are a strong function of their size, achieving a narrow particle size distribution is an important step in the development of nanoparticle applications. A promising way to do so islaser photolysis, in which a suspension of particles of several different sizes is irradiated with a high-intensity laser pulse. By carefully selecting the wavelength and energy of the pulse to match an absorption peak of one of the particle sizes (e.g., irradiating the red solution in the diagram with a 525 nm laser pulse), particles of or near that size can be selectively vaporized.
(a) A spherical silver nanoparticle of diameter D at 25°C is to be heated to its normal boiling point and vaporized with a pulsed laser. Considering the particle a closed system at constant pressure, write the energy balance for this process, look up the physical properties of silver that are required in the energy balance, and perform all the required substitutions and integrations to derive an expression for the energy Qabs(J) that must be absorbed by the particle as a function of D(nm).
(b) The total energy absorbed by a single particle (Qabs) can also be calculated from the following relation:
Qabs = FApσabs
where F(J/m2) is the energy in a single laser pulse per unit spot area (area of the laser beam) and Ap(m2) is the total surface area of the nanoparticle. The effectiveness factor, σabs, accounts for the efficiency of absorption by the nanoparticle at the wavelength of the laser pulse and is dependent on the particle size, shape, and material. For a spherical silver nanoparticle irradiated by a laser pulse with a peak wavelength of 532 nm and spot diameter of 7 mm with D ranging from 40 to 200 nm, the following empirical equation can be used for σabs.
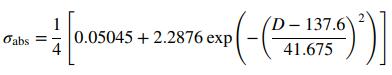
where σabs and the leading 1 4 are dimensionless and D has units of nm. Use the results of Part (a) to determine the minimum values of F required for complete vaporization of single nanoparticles with diameters of 40.0 nm, 80.0 nm, and 120.0 nm. If the pulse frequency of the laser is 10 Hz (i.e., 10 pulses per second), what is the minimum laser power P(W) required for each of those values of D? (Set up a dimensional equation relating P to F.)
(c) Suppose you have a suspension of a mixture of D = 40 nm and D = 120 nm spherical silver nanoparticles and a 10 Hz/532 nm pulsed laser source with a 7 nm diameter spot and adjustable power. Describe how you would use the laser to produce a suspension of particles of only a single size and state what that size would be.
DistributionThe word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





