The total energy eigenvalues for the hydrogen atom are given by E n = e 2 /
Question:
a.
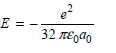
b.
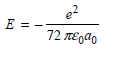
c.
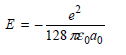
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





