Question
Given that the molar mass of NISO,-nH,0 is 262.89 g/mol, what is the value of n? [Molar mass (g/mol): Ni = 58.7, S =
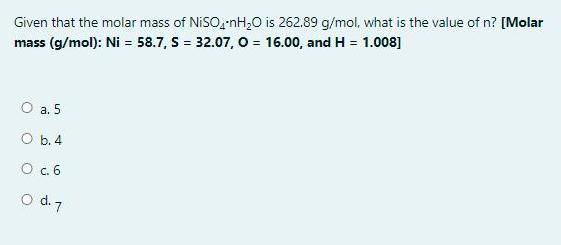
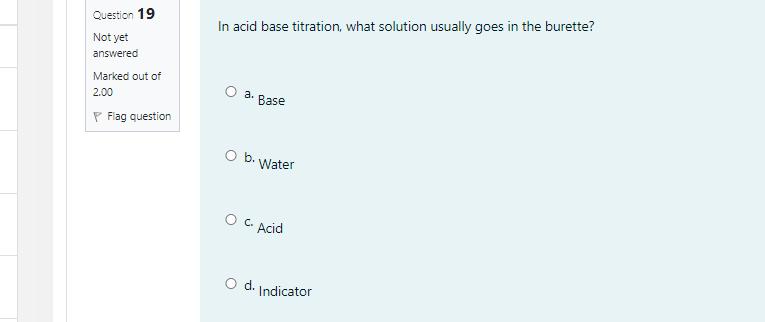

Given that the molar mass of NISO,-nH,0 is 262.89 g/mol, what is the value of n? [Molar mass (g/mol): Ni = 58.7, S = 32.07, o = 16.00, and H = 1.008] O a. 5 O b. 4 O c.6 o d. 7 Question 19 In acid base titration, what solution usually goes in the burette? Not yet answered Marked out of O a. 2.00 Base P Flag question Ob. Water O C. Acid Od. Indicator Question 18 The end point of a titration involving the reaction of MnO, to Mn2+ can be detected by Not yet the answered Marked out of 2.00 P Flag question O a. Addition of an indicator like phenolphthalien Ob. Use of litmus paper Oc. Purple color of the MnO," disappearing Od. Use of pH meter
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Q no 20 Here the value of n comes out to be 6 Henc...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



