Question: sample composition pressure temperature sample composition pressure temperature 1.0 mol Nel) 1.8 atm 259. D 1.0 mol X (s) 1.4 atm -46, B L1 mol
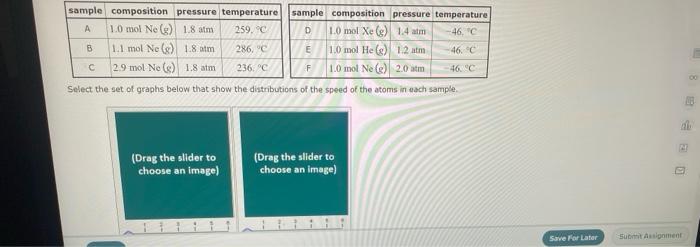
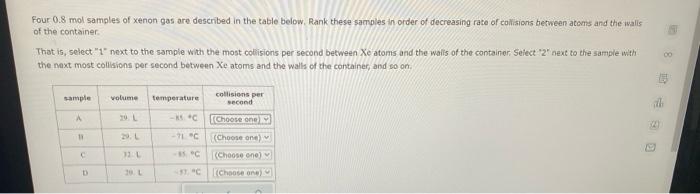
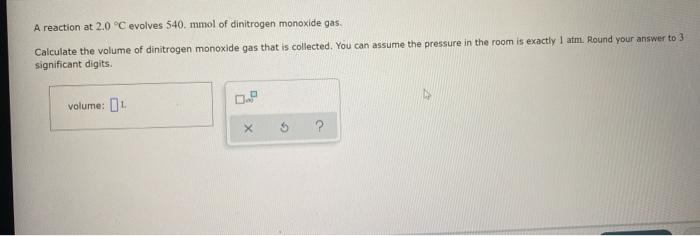
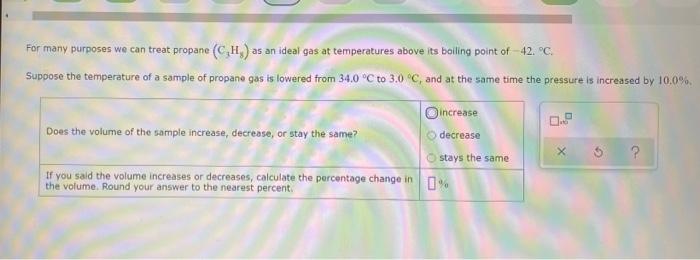
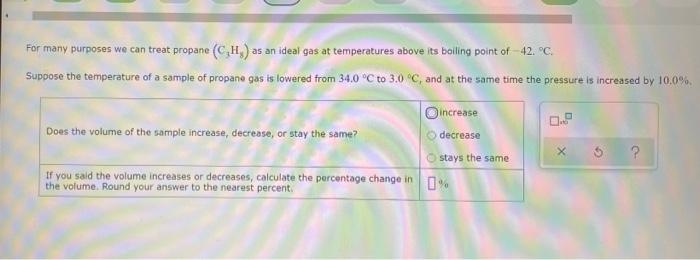

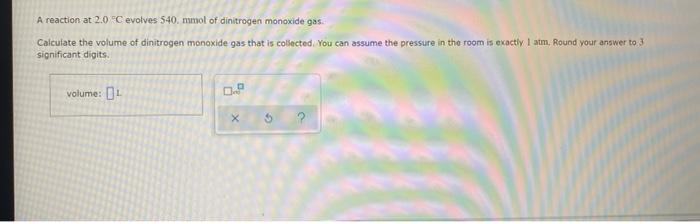
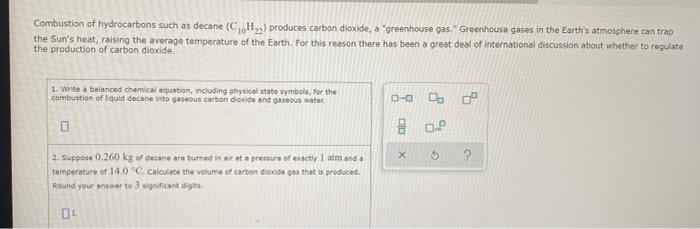
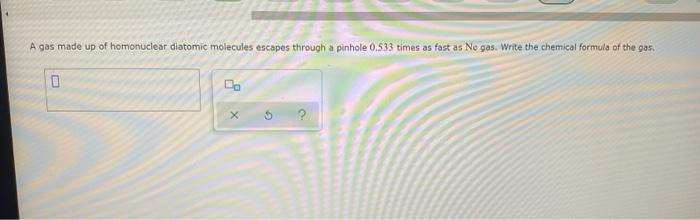

sample composition pressure temperature sample composition pressure temperature 1.0 mol Nel) 1.8 atm 259. D 1.0 mol X (s) 1.4 atm -46, B L1 mol Ne () 1.8 mm 286,"C E 1.0 mol He(e) 1.2 am 46. C 2.9 mol No ) 1.8 atm 236. "C F 1.0 mol Ne (c) 2.0 atm Select the set of graphs below that show the distributions of the speed of the atoms in each sample (Drag the slider to choose an image) (Drag the slider to choose an image) Save For Later Submit Anment Four 0.8 mol samples of xenon gas are described in the table below. Rank these samoles in order of decreasing rate of collisions between atoms and the walls of the container That is, select "1" next to the sample with the most collisions per second between Xe atoms and the walls of the container Select "2" next to the sample with the next most collisions per second between Xe atoms and the walls of the container, and so on. sample volume temperature collisions per second A [Choose on 29.4 - 71 15 Choose one) (Choose one) Choose one) D A reaction at 2.0 C evolves 540. mmol of dinitrogen monoxide gas. Calculate the volume of dinitrogen monoxide gas that is collected. You can assume the pressure in the room is exactly 1 atm. Round your answer to 3 significant digits. volume: 1) X ? For many purposes we can treat propane (CH) a as an ideal gas at temperatures above its boiling point of 42. C. Suppose the temperature of a sample of propane gas is lowered from 34.0C to 3.0 C, and at the same time the pressure is increased by 10,0% increase Does the volume of the sample increase, decrease, or stay the same? decrease stays the same If you said the volume increases or decreases, calculate the percentage change in 0% the volume. Round your answer to the nearest percent ? For many purposes we can treat propane (CH) a as an ideal gas at temperatures above its boiling point of 42. C. Suppose the temperature of a sample of propane gas is lowered from 34.0C to 3.0 C, and at the same time the pressure is increased by 10,0% increase Does the volume of the sample increase, decrease, or stay the same? decrease stays the same If you said the volume increases or decreases, calculate the percentage change in 0% the volume. Round your answer to the nearest percent ? For many purposes we can treat propane (CH) as an ideal gas at temperatures above its boiling point of 42.c, Suppose the temperature of a sample of propane gas is lowered from 34.0 C to 3.0 C, and at the same time the pressure is increased by 10.0% Does the volume of the sample Increase, decrease, or stay the same? increase decrease ? c) stays the same If you said the volume increases or decreases, calculate the percentage change in 0% the volume. Round your answer to the nearest percent A reaction at 2.0 C evolves 540. mimol of dinitrogen monoxide gas Calculate the volume of dinitrogen monoxide gas that is collected. You can assume the pressure in the room is exactly I am, Round your answer to 3 significant digits. volume: - 0.0 X 5 ? Combustion of hydrocarbons such as decane (CH) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide 1. Write a balanced chemical equation, including physical state symbols for the combustion of liquid decane into gaseous carbon dioxide and gaseous water 0.--a Da f 80P 2. Suppose 0.260 kg of decare are burned in a sta pressure of exactly I am ans. temperature of 14.0C. Calculate the volume of carbon dioxide gas that is produced Round your answer to 3 significant digits OL A gas made up of homonuclear diatomic molecules escapes through a pinhole 0.533 times as fast as Ne gas. Write the chemical formula of the gas. X 5 A gas made up of atoms escapes through a pinhole 1.25 times as fast as Xe gas. Write the chemical formula of the gas. A gas made up of homonuclear diatomic molecules escapes through a pinhole 1.49 times as fast as Cl, gas, write the chemical formula of the gas. 0 $ ? ili The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II)oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II)oxide (HgO) into liquid mercury and gaseout dioxygen 6-a da DO D. 0 3 2. Suppose 27.0 ml of dioxygen gas are produced by the reaction at a temperature of 130,0 C and pressure of exactly 1 an, calculate the mass of mercury(II)oxide that must have reacted. Round your answer to significant digits. Calculate to three significant digits the density of boron trifluoride gas at exactly 15 C and exactly atm. You can assume boron trifluoride gas behaves as an ideal gas under these conditions, IE 107 0.0 X ? il Calculate to three significant digits the density of boron trifluoride gas at exactly 15 C and exactly 1 atm. You can assume boron trifluoride gas behaves as an ideal gas under these conditions X ? Combustion of hydrocarbons such as methane (CH) produces carbon dioxide, a "greenhouse gas "Greenhouse gases in the Earth's atmosphere can trap the Sun's hest, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide 3. Webced themicaletion, incluing phycat vymotor te combustion of grous mathane Wrocarbon dioende water 0 X soppose 0.410 kg of men are umed potty Iman a temperature of 12.0C.Calculate the volume of carbon de produced Round your answer antagit DL sample composition pressure temperature sample composition pressure temperature 1.0 mol Nel) 1.8 atm 259. D 1.0 mol X (s) 1.4 atm -46, B L1 mol Ne () 1.8 mm 286,"C E 1.0 mol He(e) 1.2 am 46. C 2.9 mol No ) 1.8 atm 236. "C F 1.0 mol Ne (c) 2.0 atm Select the set of graphs below that show the distributions of the speed of the atoms in each sample (Drag the slider to choose an image) (Drag the slider to choose an image) Save For Later Submit Anment Four 0.8 mol samples of xenon gas are described in the table below. Rank these samoles in order of decreasing rate of collisions between atoms and the walls of the container That is, select "1" next to the sample with the most collisions per second between Xe atoms and the walls of the container Select "2" next to the sample with the next most collisions per second between Xe atoms and the walls of the container, and so on. sample volume temperature collisions per second A [Choose on 29.4 - 71 15 Choose one) (Choose one) Choose one) D A reaction at 2.0 C evolves 540. mmol of dinitrogen monoxide gas. Calculate the volume of dinitrogen monoxide gas that is collected. You can assume the pressure in the room is exactly 1 atm. Round your answer to 3 significant digits. volume: 1) X ? For many purposes we can treat propane (CH) a as an ideal gas at temperatures above its boiling point of 42. C. Suppose the temperature of a sample of propane gas is lowered from 34.0C to 3.0 C, and at the same time the pressure is increased by 10,0% increase Does the volume of the sample increase, decrease, or stay the same? decrease stays the same If you said the volume increases or decreases, calculate the percentage change in 0% the volume. Round your answer to the nearest percent ? For many purposes we can treat propane (CH) a as an ideal gas at temperatures above its boiling point of 42. C. Suppose the temperature of a sample of propane gas is lowered from 34.0C to 3.0 C, and at the same time the pressure is increased by 10,0% increase Does the volume of the sample increase, decrease, or stay the same? decrease stays the same If you said the volume increases or decreases, calculate the percentage change in 0% the volume. Round your answer to the nearest percent ? For many purposes we can treat propane (CH) as an ideal gas at temperatures above its boiling point of 42.c, Suppose the temperature of a sample of propane gas is lowered from 34.0 C to 3.0 C, and at the same time the pressure is increased by 10.0% Does the volume of the sample Increase, decrease, or stay the same? increase decrease ? c) stays the same If you said the volume increases or decreases, calculate the percentage change in 0% the volume. Round your answer to the nearest percent A reaction at 2.0 C evolves 540. mimol of dinitrogen monoxide gas Calculate the volume of dinitrogen monoxide gas that is collected. You can assume the pressure in the room is exactly I am, Round your answer to 3 significant digits. volume: - 0.0 X 5 ? Combustion of hydrocarbons such as decane (CH) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide 1. Write a balanced chemical equation, including physical state symbols for the combustion of liquid decane into gaseous carbon dioxide and gaseous water 0.--a Da f 80P 2. Suppose 0.260 kg of decare are burned in a sta pressure of exactly I am ans. temperature of 14.0C. Calculate the volume of carbon dioxide gas that is produced Round your answer to 3 significant digits OL A gas made up of homonuclear diatomic molecules escapes through a pinhole 0.533 times as fast as Ne gas. Write the chemical formula of the gas. X 5 A gas made up of atoms escapes through a pinhole 1.25 times as fast as Xe gas. Write the chemical formula of the gas. A gas made up of homonuclear diatomic molecules escapes through a pinhole 1.49 times as fast as Cl, gas, write the chemical formula of the gas. 0 $ ? ili The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1775. In this experiment Lavoisier found that mercury(II)oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercury(II)oxide (HgO) into liquid mercury and gaseout dioxygen 6-a da DO D. 0 3 2. Suppose 27.0 ml of dioxygen gas are produced by the reaction at a temperature of 130,0 C and pressure of exactly 1 an, calculate the mass of mercury(II)oxide that must have reacted. Round your answer to significant digits. Calculate to three significant digits the density of boron trifluoride gas at exactly 15 C and exactly atm. You can assume boron trifluoride gas behaves as an ideal gas under these conditions, IE 107 0.0 X ? il Calculate to three significant digits the density of boron trifluoride gas at exactly 15 C and exactly 1 atm. You can assume boron trifluoride gas behaves as an ideal gas under these conditions X ? Combustion of hydrocarbons such as methane (CH) produces carbon dioxide, a "greenhouse gas "Greenhouse gases in the Earth's atmosphere can trap the Sun's hest, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide 3. Webced themicaletion, incluing phycat vymotor te combustion of grous mathane Wrocarbon dioende water 0 X soppose 0.410 kg of men are umed potty Iman a temperature of 12.0C.Calculate the volume of carbon de produced Round your answer antagit DL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


