Consider LaCrSb 3 , whose structure is shown below (left). To a reasonable approximation, this structure can
Question:
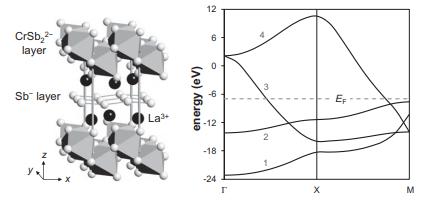
Consider LaCrSb3, whose structure is shown below (left). To a reasonable approximation, this structure can be described as independent CrSb22− layers and Sb− layers separated by La3+ ions [3]. If we neglect subtle distortions, the Sb− layer can be approximated as a 2D square lattice of Sb− ions. There is one atom per unit cell, and the Sb–Sb separation is 3.1 Å. The calculated band structure for the idealized Sb− layer is shown below, on the right.
(a) Show the orbital overlap for the Sb 5s and 5p orbitals at Γ, X, and M. Characterize the nearest-neighbor interactions for each band as (σ or π) bonding, antibonding, or nonbonding at each of these k points.
(b) Determine the orbital character of each band, numbered 1–4 in the diagram.
(c) LaCrSb3 is a metallic conductor. From the band structure above, would you expect the Sb− layers to contribute to the conductivity?
Step by Step Answer:

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt





