Consider the infinite 1D chain formed by placing boron atoms between the H 2 molecules from Problem
Question:
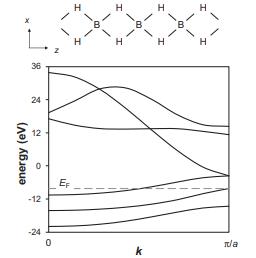
Consider the infinite 1D chain formed by placing boron atoms between the H2 molecules from Problem 6.7 to form an infinite chain (shown below) where the B–H distance is 1.27 Å while B–B and H–H are both 1.8 Å. The calculated band structure for this chain is shown below.
(a) Which of the boron orbitals can mix (form bonding/antibonding crystal orbitals) with the H2 bonding orbital, ψ+, at k = 0? Which boron orbital can mix with ψ+ at k = π/a?
(b) Which of the boron orbitals can mix with the H2 antibonding orbital, ψ−, at k = 0? Which boron orbital can mix with ψ− at k = π/a?
(c) What is the AO character of the two crystal orbitals that become degenerate at k = π/a?
(d) At k = 0, only the lowest-energy band has both B–H and H–H σ-bonding character. What is the AO character of this band at k = 0?
(e) At k = 0, the highest-energy band (E = +33.8 eV) has both B–H and H–H σantibonding character. What orbitals contribute to this band at k = 0?
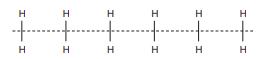
Problem 6.7
Consider an infinite 1D chain of H2 molecules where the molecular axis is oriented perpendicular to the chain direction, as shown below.
Step by Step Answer:

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt





