The UVvisible spectrum of the octahedral complex [Ni(NH 2 CH 2 CH 2 NH 2 ) 3
Question:
The UV–visible spectrum of the octahedral complex [Ni(NH2CH2CH2NH2)3]2+ is shown below.
(a) Given that ethylenediamine ligand, NH2CH2CH2NH2, is a neutral bidentate ligand (both nitrogen atoms coordinate to the metal), determine the electron configuration of the nickel ion.
(b) Use a correlation diagram to assign each of the three d-to-d transitions labeled as 1, 2, and 3 below.
(c) What color would you predict for a solution of this complex ion from the spectrum below?
(d) Compare these transitions to those observed for [Ni(H2O)6]2+ (Table 7.5) and determine which complex ion has a larger Δoct.
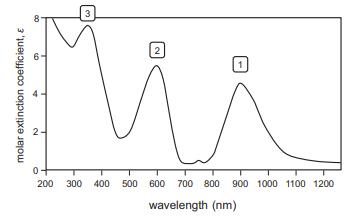
Table 7.5
![d d d d4 d6 d7 Complex ion [Ti(HO)]+ [V(HO)]* [Cr(HO)]+ 3+ [Mn(HO)]+ [Fe(HO)]+ [Co(H_O)]* [Ni(HO)]+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/9/5/1/06865aebf5c89ec01705951065203.jpg)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





