What mass of compound is present in 5.00 moles of each of the compounds in Exercise 53?
Question:
What mass of compound is present in 5.00 moles of each of the compounds in Exercise 53?
Data in Exercise 53?
Calculate the molar mass of the following substances.
a. 
b.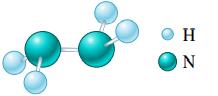
c. (NH4)2Cr2O7
Transcribed Image Text:
OH N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
To find the mass of compound present in 500 moles of each of ...View the full answer

Answered By

Monette Taban
I am currently studying Computer Science Engineering, Due to my interest in programming languages and coding, I am interesetd on Technology so I search about it read about different types of technologies, I think my this habbis will help me to solve problems of students and that is why I am signing as a question answer expert.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
What mass of compound is present in 5.00 moles of each of the compounds in Exercise 54? Data In Exercise 54? Calculate the molar mass of the following substances. a. b. Ca 3 (PO 4 ) 2 c. Na 2 HPO 4 0
-
What amount (moles) of compound is present in 1.00 g of each of the compounds in Exercise 53? Data i n Exercise 53? Calculate the molar mass of the following substances. a. b. c. (NH 4 ) 2 Cr 2 O 7 N
-
A reaction has the reaction free-energy diagram shown in Fig. P4.55. (a) Which compound is present in greatest amount when the reaction comes to equilibrium? In least amount? (b) What is the...
-
Solve each equation in Exercises. Round decimal answers to four decimal places. log 3 (x 2 + 17) - log3 (x + 5) = 1
-
Choose an appropriate forecasting technique for the data in the Excel file Coal Consumption and find the best forecasting model. Explain how you would use the model to forecast and how far into the...
-
In Example 7.2, it was determined that the sixth segment of the flat plate has the maximum power requirement. Which segment has the minimum power requirement if conditions are identical except the...
-
Figure P5.84 shows a simplified sketch of a dish-washer water supply manifold. Find the resisting torque for a water temperature of \(140^{\circ} \mathrm{F}\). \(Q=0.25 \mathrm{gal} / \mathrm{min}\)...
-
Cutthroat Company supplies flies and fishing gear to sporting goods stores and outfitters throughout the western United States. The accounts receivable clerk for Cutthroat prepared the following...
-
What is community psychology (CP)?
-
The intensity of sunlight at the Earth is about 1367W/m 2 . How much further away would the Sun have to be before the solar radiation was only as intense as a 100W light bulb 10 meters away, assuming...
-
What mass of phosphorus is present in 5.00 moles of each of the compounds in Exercise 54? Data in Exercise 54? Calculate the molar mass of the following substances. a. b. Ca 3 (PO 4 ) 2 c. Na 2 HPO 4...
-
If British pounds sell for $2 (U.S.) per pound, what should dollars sell for in pounds per dollar?
-
Plot the average number of phonons for at least five values of T to show its evolution with increasing temperatures. For each one, plot the function and show that it is a good approximation for N()...
-
Enterprise Company had the following account totals as of December 31, 20X2: Cost of goods sold $150,000 Accounts receivable 100,000 Rent revenue 10,000 Accounts payable 25,000 Sales 200,000...
-
At the beginning of the year, JKJ issued 50,000 redeemable preferred shares to the public for $5 each. The preferred shares have a dividend yield of 5%. The preferred shares must be redeemed if the...
-
One method retailers use to decide the level of marketing spend is: Responses sales revenue minus expenses. sales revenue minus expenses. mark-up minus monthly rent. mark-up minus monthly rent. gross...
-
Texas state election laws require full disclosure of "significant contributions" to campaigns. In other words, in Texas, any donated funds over what amount must be reported?
-
Sprout Inc.'s direct materials cost is $506,400, direct labor cost is $405,400, indirect materials cost is $2,250, indirect labor cost is $5,200, and other factory overhead costs are $80,700. What is...
-
Vanadium crystallizes in a body-centered cubic lattice (the V atoms occupy only the lattice points). How many V atoms are present in a unit cell?
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Show the products of thesereactions: CH NaOH Br2 Bra b) a) . .
-
The reaction of an alkenes with bromine in an alcohol as solvent produces as ether as the product. Show a mechanism for the following reaction and explain the stereochemistry of theproduct. Br . H....
-
Show all the steps in the mechanism for the formation of MTBE from methanol and isobutylene.
-
The accounts of Grand Pool Service, Inc., follow with their normal balances at April 30, 2021. The accounts are listed in no particular order. (Click the icon to view the accounts.) Read the...
-
What kind of leader do you aspire to be and what are the traits? Explain.
-
10. Create the following row vector A where it has 18 elements (1 to 18). 1 4 7 10 13 16. 11 14 17 a. Use the reshape function to obtain B: == 2 5 8 3 6 9 12 15 18 b. Create a 7 element row vector...

Study smarter with the SolutionInn App


