A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and
Question:
A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and helium mole fractions are 0.12 and 0.35, respectively. If the partial pressure of the nitrogen is 0.8 atm, determine
(a) The total pressure in the tank,
(b) The total number of moles (kmol) in the tank,
(c) The mass of the mixture in the tank.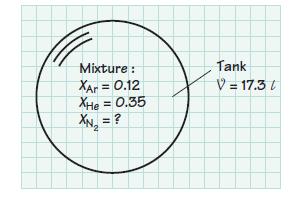
Transcribed Image Text:
Mixture : XAr = 0.12 XHe = 0.35 Tank V = 17.3 /
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To solve this problem we will use the ideal gas law which states PV nRT where P is the pressure V is the volume n is the number of moles R is the gas ...View the full answer

Answered By

Isabel Seraspi
I have experience teaching math, science, and English to students of all ages. I have also worked as a tutor in a college setting, helping students with their homework and preparing them for exams.
I believe that tutoring is a great way to help students learn. It allows students to get one-on-one help with their studies, and it gives them the chance to ask questions and get immediate feedback. Tutoring can also be tailored to the individual needs of the student, which is why I believe it is so effective.
I have seen firsthand how tutoring can help students improve their grades and confidence. I have also seen how it can help students who are struggling with a particular subject. I believe that tutoring is a great way to help students learn and succeed in school.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
1 723 Conditions for promotions 5 Years of service (Years) 6 Psychometric test (%) Required: a) LIST OF EMPLOYEES FOR PROMOTION 9 Names of employees Years of service 10 Munawarah Ali 11 Amiruddin...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
At 298 K the standard enthalpy of combustion of sucrose is -5797 k] mol-I and the standard Gibbs energy of the reaction is -6333 k] mol ". Estimate the additional non-expansion work that may be...
-
You work for a gas turbine design company and have a client who has a fairly loose specification for a gas turbine engine. You are required to design an aviation gas turbine to power the aircraft...
-
At the end of 2012, Tootsie Roll had a price- earnings ratio of 30.7, and Hershey had a price- earnings ratio of 25.3. These convert to capitalization rates of 3.25% for Tootsie Roll and 3.95% for...
-
Consider an economy that only produces and consumes two goods-food and apparel. Suppose the inflation rate based on the consumer price index is higher during the year than that based on the GDP...
-
Refer to the information in Exercise 17-4. Required 1. Compute a departmental overhead rate for the molding department based on machine hours and a department overhead rate for the trimming...
-
The Munchkin Theater is a nonprofit organization devoted to staging theater productions of plays for children in Toronto, Canada. The theater has a very small full-time professional administrative...
-
A company is planning to manufacture snowboards. The fixed costs are $129 per day and the total costs are $5,897 per daily output of 18 boards. What is the average costs per board tend to as...
-
Erika Koch is preparing her balance sheet and income statement for the month ended July 31, 2022. Use the following information to help her prepare her financial statements. Opening Balances - July...
-
Determine the total apparent specific heat at constant pressure (c p,mix in kJ/kg K) for a fuelair reactant mixture containing 1 kmol CH 4 , 2.5 kmol O 2 , and 9.4 kmol N 2 at 500 K and 1 atm. Use...
-
In Table C.2, at what reference temperature and pressure is the entropy zero? TABLE C.2 Thermodynamic Properties of Air at 1 atm* h (kJ/kg) u (kJ/kg) s (kJ/kg-K) 325.42 268.14 3.4764 335.49 275.32...
-
You are considering the possibility of pursuing a masters degree after completing your undergraduate degree. 1. List three costs (or benefits) that would be relevant to this decision, including at...
-
Q.1. Suppose today a 10 percent coupon bond sells at par. Two years from now, the required return on the same bond is 8 percent. What is the coupon rate on the bond then? What is the YTM? Q.2 A...
-
Summarize an article in which a public company is facing accounting challenges. 2. Summarize an article in which a government based organization is facing accounting challenges. 3. Summarize an...
-
For each of the two cases below, determine the water pressure, pressure head, elevation head, and total head at points A, B, and C. Please solve each case using: (a) the upper reservoir level as the...
-
Today is April 15, 2011. As the Business Manager, Linda Peysar is responsible for analyzing the current state of Tollbrook Public Library, updating the environmental scan, analyzing the various...
-
In a market demand and supply equations are: The demand curve is given as: P = 50 - 3Q The supply curve is given as: P = 10 + 2Q Assuming a perfectly competitive market: 1) What is the equilibrium...
-
Rowland & Sons Air Transport Service, Inc., has been in operation for three years. The following transactions occurred in February: Feb. 1 Paid $ 200 for rent of hangar space in February. Feb. 4...
-
The Ranch 888 Noodle Company sells two types of dried noodles:ramen, at $6.50 per box, and chow fun, at $7.70 per box. So farthis year, the company has sold a total of 110,096 boxes ofnoodles,...
-
Using the results of exercises 9 and 10, determine the mass air-to-fuel ratio (A/F) mass for the combustion of natural gas in air. (A: 17.2 kg of air/kg of fuel)
-
Determine the mass air-to-fuel ratio (A/F) mass for the combustion of an oil (represented by CH 2 ) in air.
-
Determine the mass air-to-fuel ratio (A/F)mass for the combustion of coal in air represented by the equation: CHN 0:01 O 0:1 S 0:05 + a(O 2 + 3:76N 2 ) = bCO 2 + cH 2 O + dN 2 + eSO 2
-
What role does relational calculus (or relational algebra) play in query optimization in a centralized relational database?
-
Explain three ways queries can be altered to increase database performance. Present specific examples to illustrate how implementing each query alteration could optimize the database.
-
How does the increase of data being worked on affect query optimization? What database design techniques can be applied to improve performance?

Study smarter with the SolutionInn App


