An instrument for the analysis of trace hydrocarbons in air, or in the products of combustion, uses
Question:
An instrument for the analysis of trace hydrocarbons in air, or in the products of combustion, uses a flame ionization detector. The flame in this device is fueled by a mixture of 40% (vol.) hydrogen and 60% (vol.) helium. The fuel mixture is contained in a 42.8-liter tank at 1500 psig and 298 K. The atmospheric pressure is 100 kPa. Determine
(a) The partial pressure of each constituent in the tank
(b) The total mass of the mixture. Assume ideal-gas behavior for the mixture.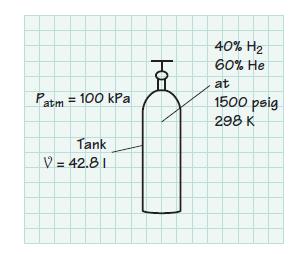
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:





