A fluid formed by pentane and hexane obeys Raoults law quite well. Such a mixture can be
Question:
A fluid formed by pentane and hexane obeys Raoult’s law quite well. Such a mixture can be used for example as the working fluid of an organic Rankine cycle power plant. The vapor pressure of pure pentane can be computed rather accurately with the Antoine equation
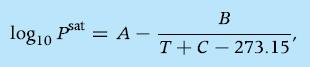
with P in bar and T in K, A = 3.978 bar, B = 1065 bar · K, and C = 232.01 K. The vapor pressure of hexane can be computed similarly, whereby the coefficients of the Antoine equation are A = 4.001 bar, B = 1171 bar · K, and C = 224.32 K.
(a) Make a P–xy chart, or phase diagram, by applying the ideal mixture model for a temperature of 30°C (a value which would be suitable for the condenser of an ORC power plant).
(b) Similarly, make a T–xy chart for a pressure of 0.5 bar.
(c) Indicate on the T–xy chart the isobaric condensation process undergone by the working fluid of an ORC power plant in the case where it is an equimolar mixture of pentane and hexane, starting from a superheated state and ending at a subcooled state. Indicate also the loci of the points giving the vapor and the liquid composition during condensation.
(d) If the overall mixture composition is equimolar, and for T = 32°C and P = 0.5 bar, determine the mass fraction of liquid, and the composition of the two phases.
(e) Compare the charts you have made with those you can obtain by using STANMIX and plot the deviations in temperature/ pressure versus the composition. What are the causes of the difference? What can be the advantage of the ideal mixture model?
Step by Step Answer:

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna





