Evaluate the specific volume of heptane for T r = 1.1 and P r = 3.5 using(a)
Question:
Evaluate the specific volume of heptane for Tr = 1.1 and Pr = 3.5 using(a) The van der Waals equation of state (Tc = 540.13 K, Pc?= 27.36 bar);(b) The generalized compressibility chart of Figure 6.6;
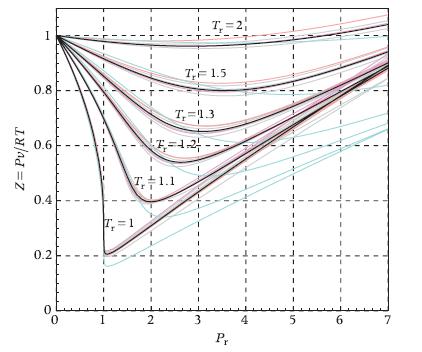
(c) TPSI. Which is more accurate among the values obtained with (a) and (b)? Consider the flow of 5 kg/s heptane in these thermodynamic conditions through a circular pipe. The flow velocity must be lower than or equal to 2 m/s in order to limit the friction losses over the long pipe. Calculate the pipe cross-sectional area using the Van der Waals equation of state and TPSI. What is the consequence of using an inaccurate thermodynamic model?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:





