The distribution of molecular velocities may be written Where P(v x )dv x is the probability that
Question:
The distribution of molecular velocities may be written
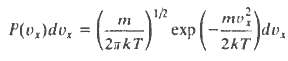
Where P(vx)dvx is the probability that a molecule will have a velocity component (in the x-direction) in the range vx to vx + dvx, m is the molecular mass, T the temperature and k Boltzmann?s constant.
From this derive the Maxwell distribution on molecular speeds (in all directions). Sketch the form of both distributions.
A vessel contains a monatomic gas at temperature T. use Maxwell?s distribution to calculate the mean kinetic energy of the molecules.
Molecules of the gas stream through a small hole into a vacuum. A box is opened for a short time and catches some of the molecules. What will be the final temperature of the gas trapped in the box? (The thermal capacity of the box is to be ignored). If
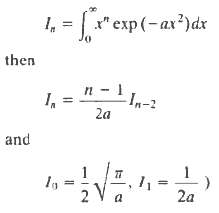
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:






