(a) Is sodium hydroxide a strong enough base to completely remove a proton from the ?-carbon acetone;...
Question:
(a) Is sodium hydroxide a strong enough base to completely remove a proton from the ?-carbon acetone; that is, does this equilibrium lie nearly completely to the right when sodium hydroxide is the base?
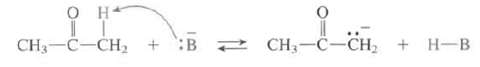
(b) Which common bases can be used to completely remove a proton from acetone?
Transcribed Image Text:
CH3-C-CH2 + :B 2 CH;-C-CH, + H-B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a The equilibrium does not lie completely to the right ...View the full answer

Answered By

Uttam Singh Bhadauriya
I've tutored high school students during my graduation years, and taught them Precalculus and Algebra for college.
I've also taught Calculus and Linear Algebra to College students.
I'm a graduate in Computer Science, but I'm highly enthusiastic about learning and teaching mathematics.
I've qualified exams like IIT-JAM and TIFR, which are entrance exams for research institutes in India.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify which of the following bases can be used to deprotonate a terminal alkyne: (a) NaOCH 3 (b) NaH (c) BuLi (d) NaOH (e) NaNH 2
-
A precipitate forms when a small amount of sodium hydroxide is added to a solution of aluminum sulfate. This precipitate dissolves when more sodium hydroxide is added. Explain what is happening.
-
Sodium hydroxide is hygroscopic-that is, it absorbs moisture when exposed to the atmosphere. A student placed a pellet of NaOH on a watch glass. A few days later, she noticed that the pellet was...
-
9. What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is...
-
Should Wagner be held responsible for these problems? Explain.
-
Each of the current-carrying wires in Figure 22-53 is long and straight, and carries the current I either into or out of the page, as shown. What is the direction of the net magnetic field produced...
-
Suppose that a forward contract on an asset is written at time zero and there are \(M\) periods until delivery. Suppose that the proportional carrying charge in period \(k\) is \(q S(k)\), where...
-
Marble Construction estimates that its WACC is 10% if equity comes from retained earnings. However, if the company issues new stock to raise new equity, it estimates that its WACC will rise to 10.8%....
-
16 ! Required information 2.77 points eBook Print References [The following information applies to the questions displayed below.] Chuck Wagon Grills, Incorporated, makes a single product-a handmade...
-
In Python 1) Write a program that asks the user to enter a string. The program should then print the following: (a) The total number of characters in the string (b) The string repeated 10 times (c)...
-
Explain which the most acidic hydrogen's in these compoundsare c) CH,CCH,CH, b) PHCH CCH3
-
Provide names for thesecompounds: b) a) , CI Br - d) ) e) CH3 NO,
-
A sealed container was filled with 0.300 mol H 2 (g), 0.400 mol I 2 (g), and 0.200 mol HI(g) at 870 K and total pressure 1.00 bar. Calculate the amounts of the components in the mixture at...
-
1. How many of each type of ticket will be sold? 2. If every patron buys popcorn and a drink, how much will this add to profit for the theatre? 3. If, in addition to the amount in #2, half the...
-
Vapocoolant sprays work on the principle of: (b) Convection (d) Evaporation (a) Conduction (c) Radiation.
-
Assume that an investor holds the following portfolio: short stock bought at a price 90$, long one 3- month maturity call option on the same stock with an exercise price of $88. a) Show the payoff...
-
Problem 3: n = 2.7 moles of an ideal gas are pumped into a chamber of volume V = 0.085 m. Randomized Variables n = 2.7 moles V=0.085 m 50% Part (a) The initial pressure of the gas is 1 atm. What is...
-
Analyze the motion of space. a. Is space static (stationary) or expanding? b. What is the proof for the motion of space? c. What does this motion do to the wavelengths of light traveling from distant...
-
In 2010, arguing that the Chinese yuan was overvalued versus the U.S. dollar, President Barack Obama said he wanted to make sure our goods are not artificially inflated in price and their goods are...
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
Of the nearly 1,300 species officially classified as endangered under the 1973 Endangered Species Act, between 450 and 500 are usually considered to be stable or improving. The remaining species are...
-
The following model is that of cholic acid, a constituent of human bile. Locate the three hydroxyl groups, and identify each as axial or equatorial, is cholic acid an A t trans steroid or an A-B...
-
Propose a biosynthetic pathway for the sesquiterpene helminthogermacrene from farnesvldiphosphate.
-
Identify the following fatty acid, and tell whether it is more likely to be found in peanut oil or in redmeat:
-
Having a bit of trouble completing part of the code for my guessing game in java. Basically, I need to add the part of the code that will allow the user to choose how many games they wish to play....
-
Find all the "daffodil numbers" between 100 and 999 and output them. "Daffodil number" refers to a three-digit number, and the cube of each digit is exactly equal to the number itself. For example,...
-
Complete the program so that each cell of array sum contains the sum of the corresponding cells of valA and valB: class Exercise3 { public static void main(String[] args) { int[] valA = {13, -22, 82,...

Study smarter with the SolutionInn App


