Exit gas from a chlorinator consists of a mixture of 20 mol% chlorine in air. This concentration
Question:
Exit gas from a chlorinator consists of a mixture of 20 mol% chlorine in air. This concentration is to be reduced to 1% chlorine by water absorption in a packed column to operate isothermally at 20°C and atmospheric pressure. Using the following equilibrium x-y data, calculate for 100 kmol/h of feed gas:
(a) The minimum water rate in kilograms per hour.
(b) NOG for twice the minimum water rate. Data for x-y at 20°C (in chlorine molefractions):
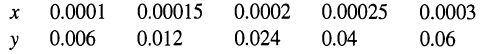
Transcribed Image Text:
0.0001 0.00025 0.0003 0.0002 0.00015 0.006 0.012 0.024 0.04 0.06 y
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (19 reviews)
Solve this problem using mole ratios Feed gas is 80 kmolh of air G and 20 kmolh of Cl 2 with Y in 2080 025 Exit gas has Y out 199 00101 X in 00 Highest value of Y in the table of equilibrium data is 0...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A gas mixture of 1 kmol carbon monoxide, 1 k mol nitrogen, and 1 k mol oxygen at 25C, 150 kPa, is heated in a constant pressure SSSF process. The exit mixture can be assumed to be in chemical...
-
A gas mixture consists of 8 kmol of H2 and 2 kmol of N2. Determine the mass of each gas and the apparent gas constant of the mixture. Answers: 16 kg, 56 kg, 1.155 kJ/kg K
-
A gas mixture consists of 5 lb mol of H2 and 4 lb mol of N2. Determine the mass of each gas and the apparent gas constant of the mixture.
-
How does the trade-off between decision management and decision control affect the form that an absorption cost system takes within a particular firm?
-
From the information in BE5-5, prepare the journal entries to record the purchase transactions on Xiaoyan Ltd.'s books, assuming a periodic inventory system is used instead of a perpetual inventory...
-
For the motor, load, and torque-speed curve shown in Figure P2.30, find the transfer function, G(s) = L (s)/E a (s). Ra N = 50 ea(t) J = 4 kg-m2 D = 8 N-m-s/rad N2 = 150 = 36 kg-m D2 = 36 N-m-s/rad...
-
For each of the following situations, calculate the \(z\)-statistic \((z)\). a. \(\mathrm{X}^{-}=8.00 ; \mu=5 ; \sigma=6 ; N=16\) b. \(\mathrm{X}^{-}=4.00 ; \mu=2 ; \sigma=8 ; N=25\) c....
-
GoGo Juice is a combination gas station and convenience store located at a busy intersection. Recently a national chain opened a similar store only a block away; consequently sales have decreased for...
-
If a project has an initial cost of $100,000 and it generates a positive cash flow of $17,000 a year for six years, what is the project internal rate of return?
-
Robertos Honey Farm in Chile makes five types of honey: cream, filtered, pasteurized, mlange (a mixture of several types), and strained, which are sold in 1 kilogram or 0.5 kilogram glass containers,...
-
Ammonia, present at a partial pressure of 12 torr in an air stream saturated with water vapor at 68F and 1 atm, must be removed to the extent of 99.6% by water absorption at the same temperature and...
-
Calculate the diameter and height for the column of Example 6.15 if the tower is packed with 1.5-in. metal Pall rings. Assume that the absorbing solution has the properties of water and use...
-
Using a fatigue allowance of 20 percent, and given the following time-study data obtained by continuous time measurement, compute the standard time.
-
We are pleased by your interest in our firm, but we are unable to extend an employment offer to you at this time. Revise the above sentence so that the bad news is de-emphasized in a dependent clause...
-
Nanette was new to her job as administrative assistant at the Barefoot Resort & Golf in Myrtle Beach, South Carolina. Alone in the office one morning, she answered a phone call from Chas, who said he...
-
We are sorry to disappoint you, but our nine-day boat excursion to the Galpagos Islands has filled up quickly and is now fully booked. We can place you on a wait list that would make you only the...
-
Act now! Revise the above refusal so that they use passive-voice instead of active-voice verbs. If possible, present the bad news positively.
-
Sleep deprivation is very common among executives and rank-and-file workers in most organizations. Hard-charging business leaders pull all-nighters or sleep only a few hours a night. When Tesla chief...
-
Define the following terms: impairment asset group traditional present value approach expected present value approach
-
The electric field due to a line charge is given by where l is a constant. Show that E is solenoidal. Show that it is also conservative. E =
-
There are a surprising number of otherwise normal people who consult psychics for advice about how to live their lives. Explain how believing in someone who appears to have psychic ability might...
-
In an energy balance, what are the two most common references (datums) used for enthalpy and entropy? Does one have an advantage over the other?
-
Give examples of separation operations used for the steps in a bioprocess.
-
Prove that, in a triangular diagram where each vertex represents a pure component, the composition of the system at any point inside the triangle is proportional to the length of the respective...
-
Pangasa Co. paid its annual worker accident insurance premium of $48,000 on its manufacturing facility in January. The company expects two worker accidents to happen during the year, and to make...
-
3. The game of baseball is often analyzed using Markov models. The state of the game can be represented by listing bases that have runners and keeping track of how many outs there are. There are...
-
Thornton Electronics currently produces the shipping containers it uses to deliver the electronics products it sells. The monthly cost of producing 9,300 containers follows. Unit-level materials...

Study smarter with the SolutionInn App


