a. The osmotic coefficient of a solvent S is defined by the relation where S
Question:
a. The osmotic coefficient of a solvent ∅S is defined by the relation
![]()
where ∅S → 1 as xS → 1. Develop an expression relating the activity coefficient γS to the osmotic coefficient ∅S.
b. Robinson and Wood report the following interpolated values for the osmotic coefficient of seawater as a function of concentration at 25°C.
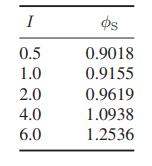
Here I is the ionic strength, defined as
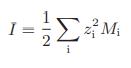
where zi is the valence of the ith ionic species and Mi is its molality. For simplicity, seawater may be considered to be a mixture of only sodium chloride and water. Compute the water activity coefficient and the equilibrium osmotic pressure for each of the solutions in the table.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





