Question: The bond enthalpy in NO is 632 kJ mol 1 and that of each NO bond in NO 2 is 469 kJ mol
The bond enthalpy in NO is 632 kJ · mol–1 and that of each N—O bond in NO2 is 469 kJ · mol–1. Using Lewis structures and the data in Table 4E.3, explain
(a) The difference in bond enthalpies between the two molecules;
(b) The fact that the bond enthalpies of the two bonds in NO2 are the same.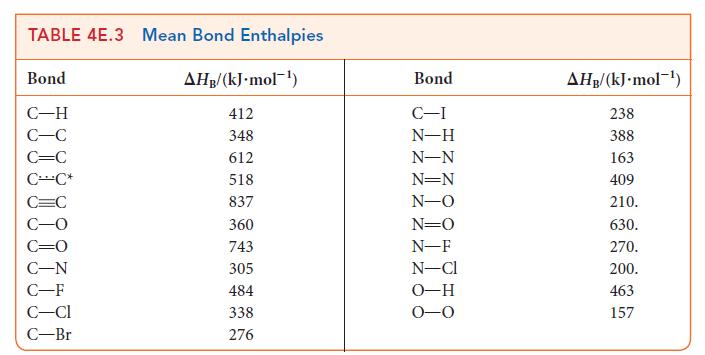
TABLE 4E.3 Mean Bond Enthalpies Bond C-H C-C C=C CC* C=C C-N C-F C-Cl C-Br AHB/(kJ.mol-) 412 348 612 518 837 360 743 305 484 338 276 Bond C-I N-H N-N N=N N-O N=O N-F N-Cl O-H 0-0 AHB/(kJ.mol-) 238 388 163 409 210. 630. 270. 200. 463 157
Step by Step Solution
3.21 Rating (154 Votes )
There are 3 Steps involved in it
a The difference in bond enthalpies between NO and NO can be attributed to the nature of the bonds and the molecular structure of each species For NO ... View full answer

Get step-by-step solutions from verified subject matter experts


