The bond enthalpy in NO is 632 kJ mol 1 and that of each NO bond
Question:
The bond enthalpy in NO is 632 kJ · mol–1 and that of each N—O bond in NO2 is 469 kJ · mol–1. Using Lewis structures and the data in Table 4E.3, explain
(a) The difference in bond enthalpies between the two molecules;
(b) The fact that the bond enthalpies of the two bonds in NO2 are the same.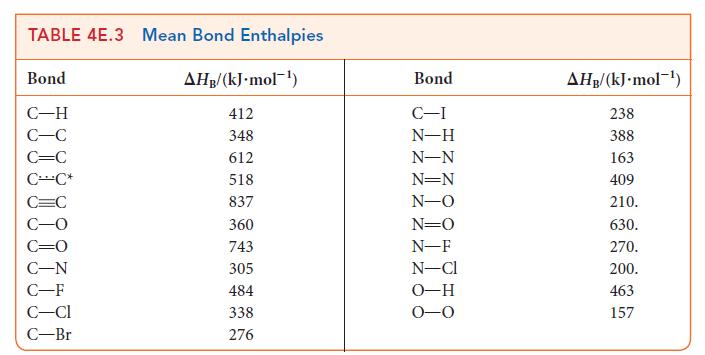
Transcribed Image Text:
TABLE 4E.3 Mean Bond Enthalpies Bond C-H C-C C=C CC* C=C C-N C-F C-Cl C-Br AHB/(kJ.mol-¹) 412 348 612 518 837 360 743 305 484 338 276 Bond C-I N-H N-N N=N N-O N=O N-F N-Cl O-H 0-0 AHB/(kJ.mol-¹) 238 388 163 409 210. 630. 270. 200. 463 157
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a The difference in bond enthalpies between NO and NO can be attributed to the nature of the bonds and the molecular structure of each species For NO ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The nitrogen oxides are common pollutants generated by internal combustion engines and power plants. They not only contribute to the respiratory distress caused by smog but, if they reach the...
-
In 1998 scientists using a special type of electron microscope were able to measure the force needed to break a single chemical bond. If 2.0 X 10-9 N was needed to break a C-Si bond, estimate the...
-
A discrete random process, X[n], is generated by repeated tosses of a coin. Let the occurrence of a head be denoted by 1 and that of a tail by - 1. A new discrete random process is generated by Y...
-
Please answer the following. Show your calculations for potential partial credit. Assume the expected return on the market is 14 percent and the risk-free rate is 4 percent. A.)What is the expected...
-
You are the management accountant for Newberry Manufacturing. Your company does custom carpentry work and uses a job order cost accounting system. Newberry sends detailed job cost sheets to its...
-
Find the required horizontal and vertical components of the given vectors. Two upward forces are acting on a bolt. One force of 60.5 lb acts at an angle of 82.4 above the horizontal, and the other...
-
(a) Use Figure \(7.2 a\) to calculate the kinetic energies of the two carts at \(t=30,60\), and \(90 \mathrm{~ms}\). (b) What is the kinetic energy of the system at each instant? Figure 7.2...
-
Daphne Kavier is the managing director of the Topeka Day Care Center. Topeka is currently set up as a full-time child care facility for children between the ages of 12 months and 6 years. Daphne is...
-
"Managing Away Bad Habits Team Assignment Organizational Behavior IILeadership Assigned is ashort case from the exercise Managing Away Bad Habits. The task is to develop a turnaround strategy for...
-
Ethanol is a renewable and clean-burning component of gasoline: What is the change in internal energy for the reaction of 1.00 mol C 2 H 5 OH(l)? 2 CHOH(I) + 602(g) - 4 CO2(g) + 6 H,O(1) -1368 kJ
-
The standard enthalpy of reaction of N 2 (g) + 3 H 2 (g) 2 NH 3 g) is 92.22 kJ mol 1 at 298 K. The industrial synthesis takes place at 450. C. What is the standard reaction enthalpy at the...
-
Find the singular values of the given matrix. [V2 A = V2.
-
The rights of shareholders are established only in the articles of incorporation. (True/False)
-
Management serves as the representative of the employees in bargaining with the union over the rights of the employees. (True/False)
-
In a consolidation, one corporation acquires all the assets and liabilities of another corporation, which then ceases to exist. (True/False)
-
Why do companies need to be careful about whom they hire and how much they supervise or monitor their employees?
-
What are the effects of a partners dissociation from a partnership?
-
Draw a standard supply and demand diagram for televisions, and indicate the equilibrium price and output. a. Assuming that the production of televisions generates external costs, illustrate the...
-
1) Predict the organicproduct formed when BzCl reacts with cyclohexanol. BzCl = benzoylchloride. 2) Provide the majororganic product of the reaction below. 3) Draw the structureof the product formed...
-
At a particular temperature, K = 3.75 for the reaction SO2(g) + NO2(g) SO3(g) + NO(g) If all four gases had initial concentrations of 0.800 M, calculate the equilibrium concentrations of the gases.
-
At 25oC, K = 0.090 for the reaction H2O(g) + Cl2O(g) 2HOCl(g) Calculate the concentrations of all species at equilibrium for each of the following cases. a. 1.0 g of H2O and 2.0 g of Cl2O are mixed...
-
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in a 5.00-L rigid container are [H2] = 0.0500 M, [F2] = 0.0100 M, and [HF] = 0.400 M. H2(g) + F2(g) ...
-
Page 144 5-12. Jefferson County established a capital project fund in 2023 to build low-income housing with the transfer of $100,000 from the General Fund. A portion of that was expended on...
-
Current Attempt in Progress Vin Diesel owns the Fredonia Barber Shop. He employs four barbers and pays each a base salary of $1,250 per month. One of the barbers serves as the manager and receives an...
-
Ragesun Corporation issued 510 shares of $10 par value common stock and 153 shares of $54 par value preferred stock for a lump sum of $22,950. The common stock has a market price of $20 per share,...

Study smarter with the SolutionInn App


