Question: Ethanol is a renewable and clean-burning component of gasoline: What is the change in internal energy for the reaction of 1.00 mol C 2 H
Ethanol is a renewable and clean-burning component of gasoline: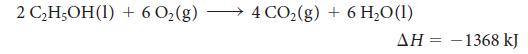
What is the change in internal energy for the reaction of 1.00 mol C2H5OH(l)?
2 CHOH(I) + 602(g) - 4 CO2(g) + 6 H,O(1) -1368 kJ
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
The change in internal energy AH for the given reaction is 1368 kJmol The relationship between the c... View full answer

Get step-by-step solutions from verified subject matter experts


