The decomposition of ozone in the stratosphere is an issue of great concern because stratospheric ozone protects
Question:
The decomposition of ozone in the stratosphere is an issue of great concern because stratospheric ozone protects life on Earth. Suppose you are studying the mechanism of ozone decomposition. The following rate law has been determined for the decomposition of ozone discussed at the beginning of this Topic,![2 03(g) 3 0(g) [03] [0] Rate of decomposition of O3 = k,-](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1703/9/4/4/399659020cf2669b1703944398822.jpg)
The following mechanism has been proposed: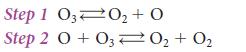
Measurements of the rates of the elementary forward reactions show that the slow step is the forward reaction in the second step, the attack of O on O3. Its reverse, O2 + O2 → O + O3, is so slow that it can be ignored. Derive the rate law implied by the mechanism, and confirm that it matches the observed rate law.
A Note on Good Practice: Be careful to distinguish the sign ⇄ (paired regular arrows), which signifies only that both forward and reverse reactions may occur, from the sign ⇄ (paired single-barbed arrows), which signifies that the reactions are at equilibrium.
PLAN Write the rate laws for the elementary reactions and combine them into the overall rate law. If necessary, use the steady-state approximation for any intermediates.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





