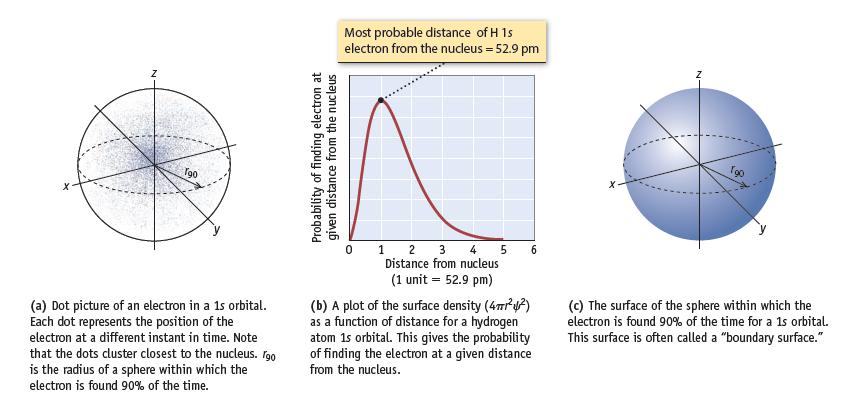
Figure 6.12b shows the probability of finding a hydrogen 1s electron at various distances from the nucleus.
Question:
Figure 6.12b shows the probability of finding a hydrogen 1s electron at various distances from the nucleus. To create the graph in this figure, the electron cloud is first divided into a series of thin concentric shells about the nucleus and then the probability of finding the electron in each shell is evaluated. The volume of each shell is given by the equation V = 4πr2(d), where d is the thickness of the shell and r is the distance of the shell from the nucleus. The probability of finding the electron in each shell is Probability = 4πr2ψ2(d) where ψ is the 1s wavefunction for hydrogen (ao in this equation is 52.9 pm).
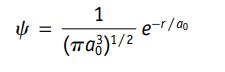 (a) The most probable distance for a 1s electron in the hydrogen atom is at 52.9 pm. Evaluate the probability of finding the electron in a concentric shell 1.0 pm thick at this distance from the nucleus.
(a) The most probable distance for a 1s electron in the hydrogen atom is at 52.9 pm. Evaluate the probability of finding the electron in a concentric shell 1.0 pm thick at this distance from the nucleus.
(b) Calculate the probability of finding the electron in a shell 1.0 pm thick at distances from the nucleus of 0.50 ao and 4 ao. Compare the results with the probabilities at ao. Are these probabilities in accord with the surface density plot shown in Figure 6.12b?
Data given in Figure 6.12
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





