This problem re-examines Example 15-5 by considering the effect of changing the pressure. A. Re-do Example 15-5.
Question:
This problem re-examines Example 15-5 by considering the effect of changing the pressure.
A. Re-do Example 15-5. Use P = 49 bar for all pressures throughout the system, assume all mixtures are ideal solutions of real gases, and assume all other specifications are the same as in Example 15-5, part B.
B. Use relevant system parameters to quantify the advantages and disadvantages of a system pressure P = 49 bar compared to P = 25 bar.
Example 15-5.
30 mol/s of hydrogen gas and 15 mol/s of air, each compressed to 25 bar, enter a steadystate reactor as shown in Figure 15-5, where the nitrogen in the air reacts with the hydrogen to form ammonia
N2 + 3H2 ↔2NH3
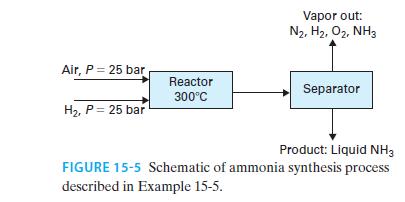
The stream leaving the reactor is at equilibrium at a temperature of 300°C. It is then cooled (though it remains at 25 bar) and sent to a vessel in which the liquid and vapor are separated from each other. The liquid ammonia is the desired product. Find the flow rate of liquid product and the composition and flow rate of the vapor stream for the following.
A. The streams leaving the separator are in VLE at 25°C.
B. The streams leaving the separator are in VLE at 0°C.
Model air as 79 mol% nitrogen and 21 mol% oxygen, model all gas/vapor phases as ideal, and assume the liquid product is pure ammonia (in other words, nitrogen, oxygen, and hydrogen are modeled as gases that are insoluble in ammonia).
There is no catalyst in the separator, so it can be assumed the reaction occurs in the reactor only
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





