This problem considers the ammonia synthesis process described in Example 15-5, part B. The fresh air and
Question:
This problem considers the ammonia synthesis process described in Example 15-5, part B. The fresh air and hydrogen each enter the process at T = 25°C and P = 1 bar, and need to be compressed to P = 25 bar. This is done in two compressions stages, with the gas leaving the first compressor cooled to T = 25°C and P = 5 bar before it enters the second stage. Assume all compressors have п = 0.8.
A. Find the rate at which work is added to each compressor, and the rate at which heat is removed in the interstage cooler for the hydrogen.
B. Find the rate at work is added to each compressor, and the rate at which heat is removed in the interstage cooler, for the air.
C. Find the rate at which heat must be added to (or removed from) the reactor in order to maintain its constant temperature of 300°C.
Use an ideal gas model for all streams that are at pressures below 10 bar, and model streams above 10 bar as ideal solutions of real gases. State and ex plain any other assumptions that you make.
Example 15-5.
30 mol/s of hydrogen gas and 15 mol/s of air, each compressed to 25 bar, enter a steadystate reactor as shown in Figure 15-5, where the nitrogen in the air reacts with the hydrogen to form ammonia
N2 + 3H2 ↔2NH3
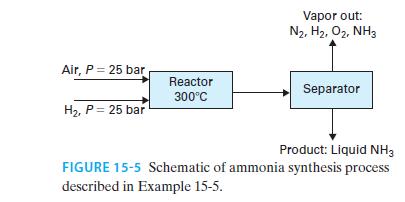
The stream leaving the reactor is at equilibrium at a temperature of 300°C. It is then cooled (though it remains at 25 bar) and sent to a vessel in which the liquid and vapor are separated from each other. The liquid ammonia is the desired product. Find the flow rate of liquid product and the composition and flow rate of the vapor stream for the following.
A. The streams leaving the separator are in VLE at 25°C.
B. The streams leaving the separator are in VLE at 0°C.
Model air as 79 mol% nitrogen and 21 mol% oxygen, model all gas/vapor phases as ideal, and assume the liquid product is pure ammonia (in other words, nitrogen, oxygen, and hydrogen are modeled as gases that are insoluble in ammonia).
There is no catalyst in the separator, so it can be assumed the reaction occurs in the reactor only
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





