Give the oxidation state of each element in the following compounds and ions; Pauling electronegativity values in
Question:
Give the oxidation state of each element in the following compounds and ions; Pauling electronegativity values in Appendix 7 may be useful:
(a) CaO;
(b) H2O;
(c) HF;
(d) FeCl2;
(e) XeF6;
(f) OsO4;
(g) Na2SO4;
(h) [PO4]3−;
(i) [PdCl4]2−;
(j) [ClO4]−;
(k) [Cr(OH2)6]3+
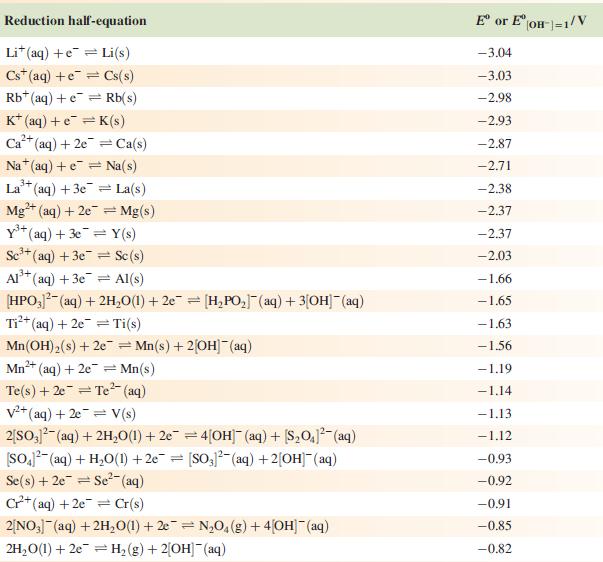
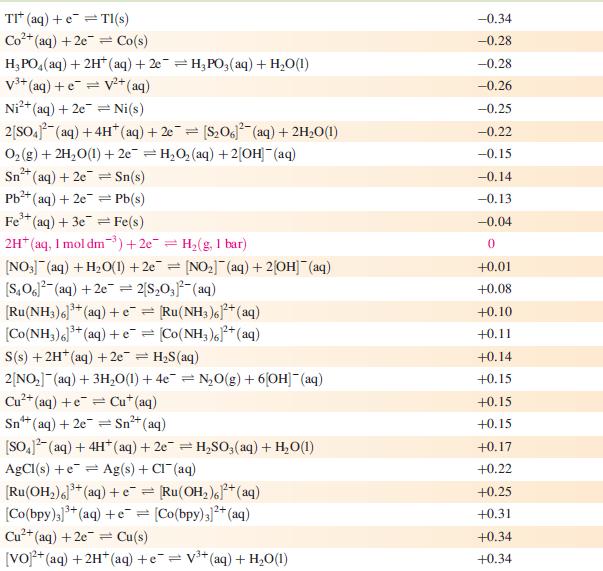
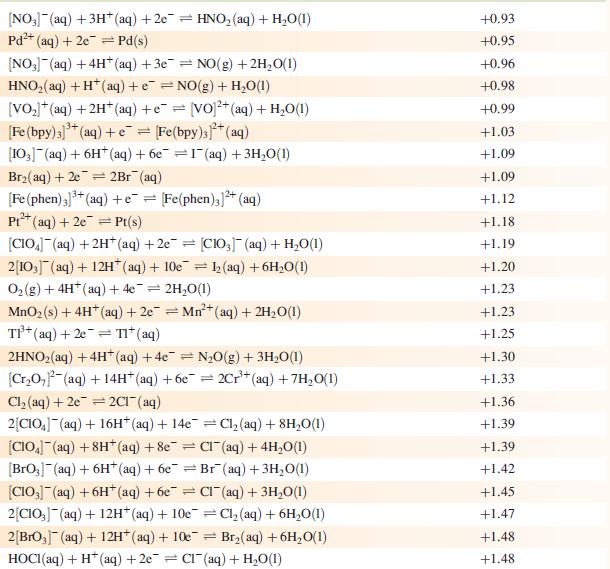
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of Eo at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of E° refers to [OH−] = 1 mol dm−3, hence the notation E°[OH−] = 1

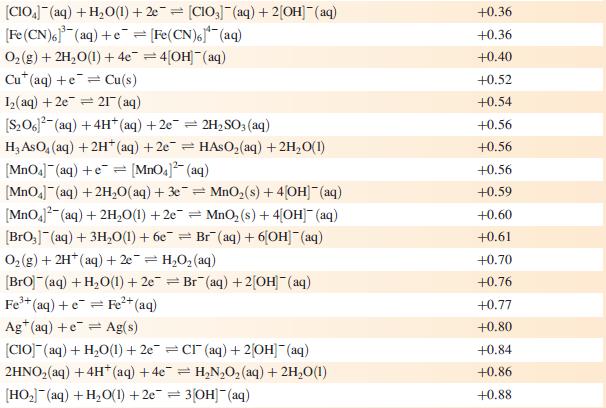

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





