Second order rate constants, k 2 , for the reaction of trans-[Pt(PEt 3 ) 2 Ph(MeOH)] +
Question:
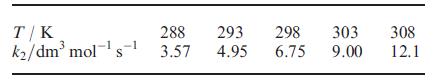
Second order rate constants, k2, for the reaction of trans-[Pt(PEt3)2Ph(MeOH)]+ with pyridine (py) in MeOH to give trans-[Pt(PEt3)2Ph(py)]+ vary with temperature as shown below. Use the data to determine the activation enthalpy and activation entropy for the reaction.
Transcribed Image Text:
T/K 288 293 298 k₂/dm³ mol-¹s¹ 3.57 4.95 6.75 303 9.00 308 12.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine the activation enthalpy H and activation entropy S for a ...View the full answer

Answered By

Vishal madan
Experienced in Science is now likely available on social media here, only for you guys to explore my thoughts, thinking, opinions, and much more to guide humanity. My name is Hammad Shaukat. My greatest passion in life is teaching. I was born and raised in Pakistan (Rawalpindi) and experienced great success at school and in my higher education due to amazing and unforgettable teachers. This is the foundation of my commitment to helping my students, whatever their abilities may be. Currently, I am studying for a bachelor's degree specializing in physics. I have been tutoring and teaching for 2 years in various settings: tutoring small and large groups, private individual tutoring, and teaching in rural, suburban, and urban classroom and home settings. I am specializing in physics. I have much experience in science and am interested in astrology and cosmology. For instructors and tutors, you can mail at any time. ( SPECIALIZED IN SCIENCES ) Major subjects are sciences.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the average Cp value in KJ/kg-K of a gas if 522 KJ/kg of heat is necessary to raise the temperature from 3OOK to 800K making the pressure constant * 1.038 1.026 1.440 1.044 What is the...
-
The equilibrium constant for the reaction of NH3 (aq) + H2O NH+4 + OH- is Kb = 1.479 10-5 at 5C 1.570 10 -5 at 10oC (a) Assuming H and S are constant in the interval 5 - 10C (probably a good...
-
The enthalpy of neutralization for the reaction of a strong acid with a strong base is 256 kJ/ mol of water produced. How much energy will be released when 200.0 mL of 0.400 M HNO3 is mixed with...
-
Explain Computer Network Types.
-
Xeriscape Nurseries, Inc., has four divisions. The corporations controller has been asked to prepare a cash budget for the Northern Division for the first quarter. Projected data supporting this...
-
Explain what a derived unit of measure is.
-
A project has been selected for implementation. The net cash flow (NCF) profile associated with the project is shown below. MARR is 10 percent/year. a. What is the annual worth of this investment? b....
-
R. C. Coleman distributes a variety of food products that are sold through grocery store and supermarket outlets. The company receives orders directly from the individual outlets, with a typical...
-
When a company lends money to employees at a rate of 4%, the company will record ______. Multiple choice question. a liability called Accounts Payable a liability called Notes Payable an asset called...
-
What structural features would you expect in the solid state of (a) Cs 2 [NpO 2 (acac) 3 ], (b) Np(BH 4 ) 4 , (c) The guanidinium salt of [ThF 3 (CO 3 ) 3 ] 5 , (d) Li 3 [LuMe 6 ]3DME, (e) Sm{CH(SiMe...
-
Prepare compound journal entries for each transaction. a. The owner, J. Cruz, invests $6,500 cash and $3,500 of equipment in the company. b. The company acquires $2,000 of supplies by paying $500...
-
We have discussed linear and exponential trend lines. Another popular choice is a power trend line, also called a constant elasticity trend line. This trend line has the form y = ax b , and it has...
-
When the + 2 . 0 0 C point charge moves 0 . 6 0 0 m to the right, what is the change in the electric potential energy of the plate / charge system?
-
A major difficulty that businesses face in increasing their technology is the financial aspect of supplying the new technology to all that require it, and then providing the necessary training. Can...
-
Alternatives High Demand Moderate Demand Low Demand Add a new building 50,000 9,000 30,000 Extend hours for existing building 25,000 5,150 5,000 Do Nothing 0 0 0 Step 3 of 3: What would Gregor's...
-
This hypothetical exercise simulates Part B of the end of semester assessment. Part B is worth 30 marks and the marks are split as follows: Identification of issue/s (5 marks), Relevant rule/s of law...
-
What are the challenges with the current CVS pharmacy service process? How would you redesign the CVS pharmacy service process (you may provide one or more relevant graphics in an appendix)? Explain...
-
Refer to Exercise 8.101. a. Find b for each of the following values of the population mean: 49, 47, 45, 43, and 41. b. Plot each value of b you obtained in part a against its associated population...
-
What is an insurable interest? Why is it important?
-
Using toluene as your only source of carbon atoms, show how you would prepare the following compound.
-
Starting with isopropyl benzene, propose a synthesis for acetophenone.
-
Propose a plausible synthesis for the following transformation.
-
An electronics firm decides to launch two new models of computer, COM1 and COM2. The cost of producing each machine of type COM1 is $1200 and the cost for COM2 is $1600. The firm recognizes that it...
-
Both financial and managerial accounting are important to a company's success. What do you think contributes more to this success and why?
-
2. The Competition Commission are concerned about a lack of competition amongst cellular providers in South Africa. Suppose they issue licenses to 10 smaller cellular providers with the aim of...

Study smarter with the SolutionInn App


