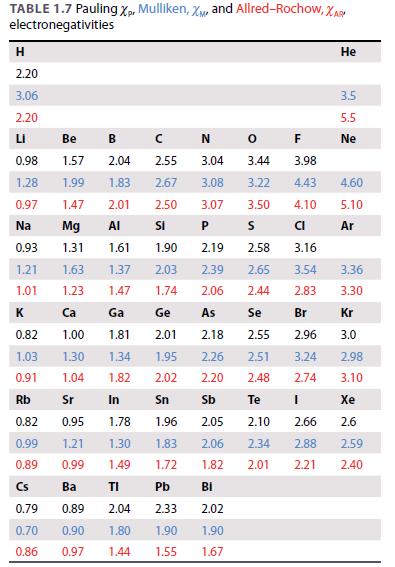
Use the Ketelaar triangle in Fig. 2.28 and the electronegativity values in Table 1.7 to predict what
Question:
Use the Ketelaar triangle in Fig. 2.28 and the electronegativity values in Table 1.7 to predict what type of bonding is likely to dominate in
(a) BCl3,
(b) KCl,
(c) BeO.
Figure 2.28.
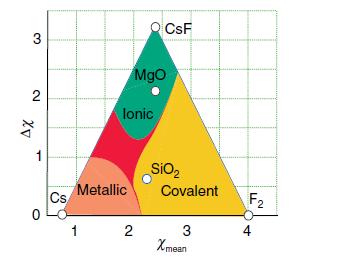
Table 1.7.
Transcribed Image Text:
3 2 ΔΧ 1 0 Cs Metallic 1 lonic MgO CsF 2 SiO₂ Covalent 3 Xmean F₂ 4 N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To predict the type of bonding likely to dominate in the given compounds BCl 3 KCl and BeO we can use the Ketelaar triangle and the electronegativity ...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:
Students also viewed these Sciences questions
-
What type of bonding does KCl have? Fully explain your reasoning by referring to the electronic structure and electronic properties of each element.
-
Consider the following information about the newly discovered element, vulcium, whose symbol is Vu. Vulcium is a solid at room temperature. It is easily cut by a penknife to reveal a shiny surface...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Net income Depreciation expense Accounts receivable increase (decrease) Inventory increase (decrease) Accounts payable increase (decrease) Accrued liabilities increase (decrease) O Changes in current...
-
Las Paletas Corporation has two different bonds currently outstanding. Bond M has a face value of $20,000 and matures in 20 years. The bond makes no payments for the first six years, then pays $1,100...
-
What are the chances that microbial life forms might one day be found elsewhere in our solar system? How much effort should we exert in searching for such life forms? And what precautions should we...
-
Tom Bonacci brought his Jeep to Brewer Service Station to investigate a strange noise the vehicle was making. The Jeep was raised up on an automobile lift so that Brewer employee Paul Gebing could...
-
Duncan Company combines its operating expenses for budget purposes in a selling and administrative expense budget. For the first 6 months of 2012, the following data are available. 1. Sales: 20,000...
-
5. A compact car has a maximum acceleration of 4.5 m/s when it only carries the driver. The driver and the car combined, have a total mass of 1250 kg. What is the maximum acceleration after picking...
-
Rationalize the bond dissociation energy (D) and bond length data of the gaseous diatomic species given in the following table and highlight the atoms that obey the octet rule. C BN NF BeO D/(kJ...
-
Use the data in Table 2.7 to calculate the standard enthalpy of the reaction 2 H 2 (g) + O 2 (g) 2 H 2 O(g). The experimental value is 484 kJ. Account for the difference between the estimated and...
-
A standard lookback call option on stock has a value at maturity equal to (Value of the stock at maturity - Minimum value of stock during the life of the option prior to maturity) or $0, whichever is...
-
How Artificial Intelligence can be used to improve returning in supply chain? Explain
-
Discuss the mechanisms and algorithms employed in disk scheduling within operating systems to optimize I/O performance. How do approaches like FCFS (First-Come, First-Served), SSTF (Shortest Seek...
-
Explain what is meant by internal and external environments. Provide examples by Opsens company.
-
Over 7 years ago your company built a restaurant in New London, Connecticut. It was completed in 2016 with a total cost of $4,940,000. You were awarded a contract for a similar restaurant (almost...
-
Assume a 10% discount rate. Which among the choices below have the highest value, A or B, if any? Show work. A.$1,000 per year for perpetuity (first payment at the end of the first period). B.$10,000...
-
A paralegal at the Vermont State Attorney Generals office wants to know how many companies in Vermont provide health insurance benefits to all employees. She chooses 12 companies at random and finds...
-
What is EBIT/eps analysis? What information does it provide managers?
-
Table 5.6 gives the results of a self-consistent field (SCF) quantum chemical calculation for H 2 O using an orbital basis set of the atomic orbitals of O and the LGOs of an H---H fragment. The axis...
-
Using Figs. 5.22, 5.23 and 5.25 to help you, compare the MO pictures of the bonding in BF 3 and [NO 3 ]. What approximations have you made in your bonding analyses? Figure 5.22. Figure 5.23 Figure...
-
In problems 4.28 to 4.51, refer to Table 4.3 for isotopic abundances where needed. NaBH 4 contains the tetrahedral [BH4] ion. Although NaBH 4 hydrolyses slowly in water, it is possible to obtain a...
-
Open and Read the link document below + ASK one single question about the article, and Respond to it. Also, include a "Source" or "Reference" at the end of your paragraph:...
-
Assume you have been put in charge of a new task force to determine the cause of lost sales in the Western region of your plastics manufacturing firm. As the leader of the task force, it is your job...
-
6. In a hydropower plant the pressure is measured at the start, and end of the draft tube as shownin the picture below. P1 L R P2 The draft tube has the following dimensions R = 4m, R2 = 6m, L = 12m,...

Study smarter with the SolutionInn App


