Using data from Appendix 11, and the value for the standard Gibbs energy of formation for PbS
Question:
Using data from Appendix 11, and the value for the standard Gibbs energy of formation for PbS of −99 kJ mol–1, determine a value for Ksp for this salt.
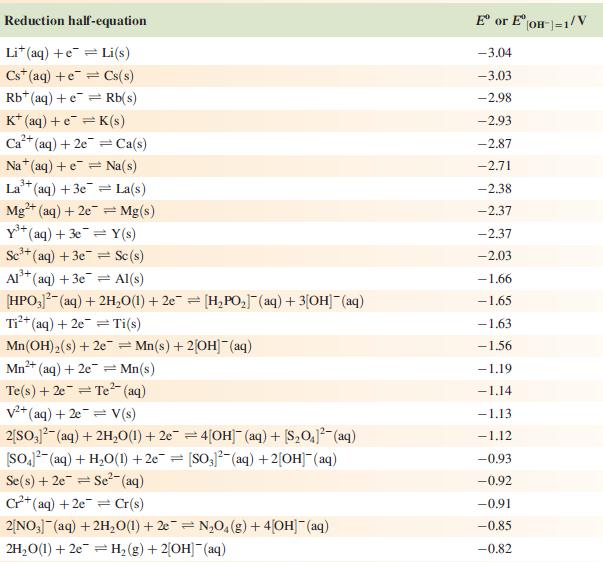
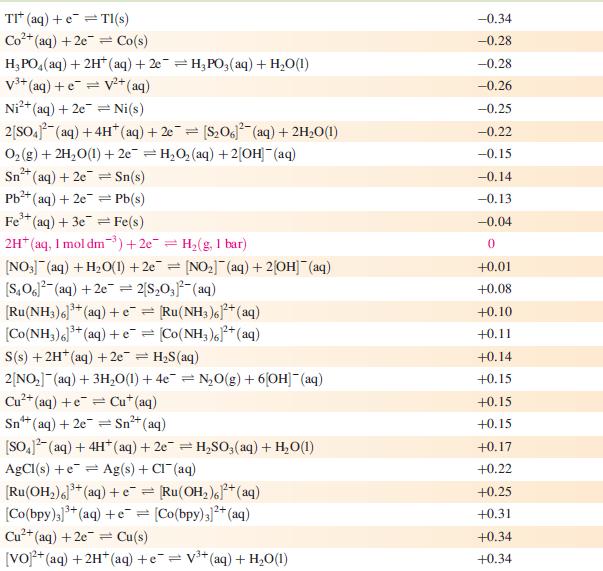
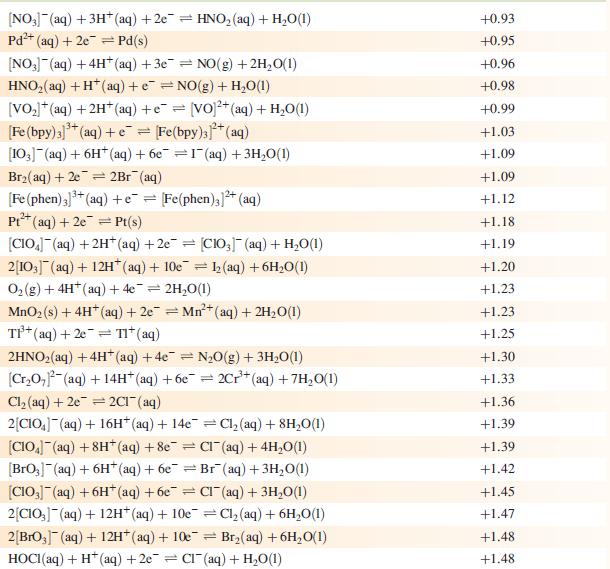
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of E° at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of E° refers to [OH−] = 1 mol dm−3, hence the notation E°[OH−] = 1

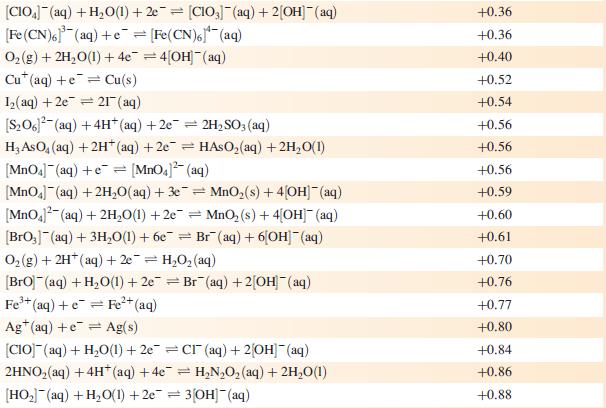

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





