Consider the following reaction: The Gibbs energy of reaction at 298 K is determined to be and
Question:
Consider the following reaction: ![]()
The Gibbs energy of reaction at 298 K is determined to be ![]() and at a given temperature, the equilibrium constant is reported to be KT = 16. Now consider the reaction is written as follows:
and at a given temperature, the equilibrium constant is reported to be KT = 16. Now consider the reaction is written as follows: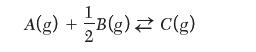
What are the values of![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





